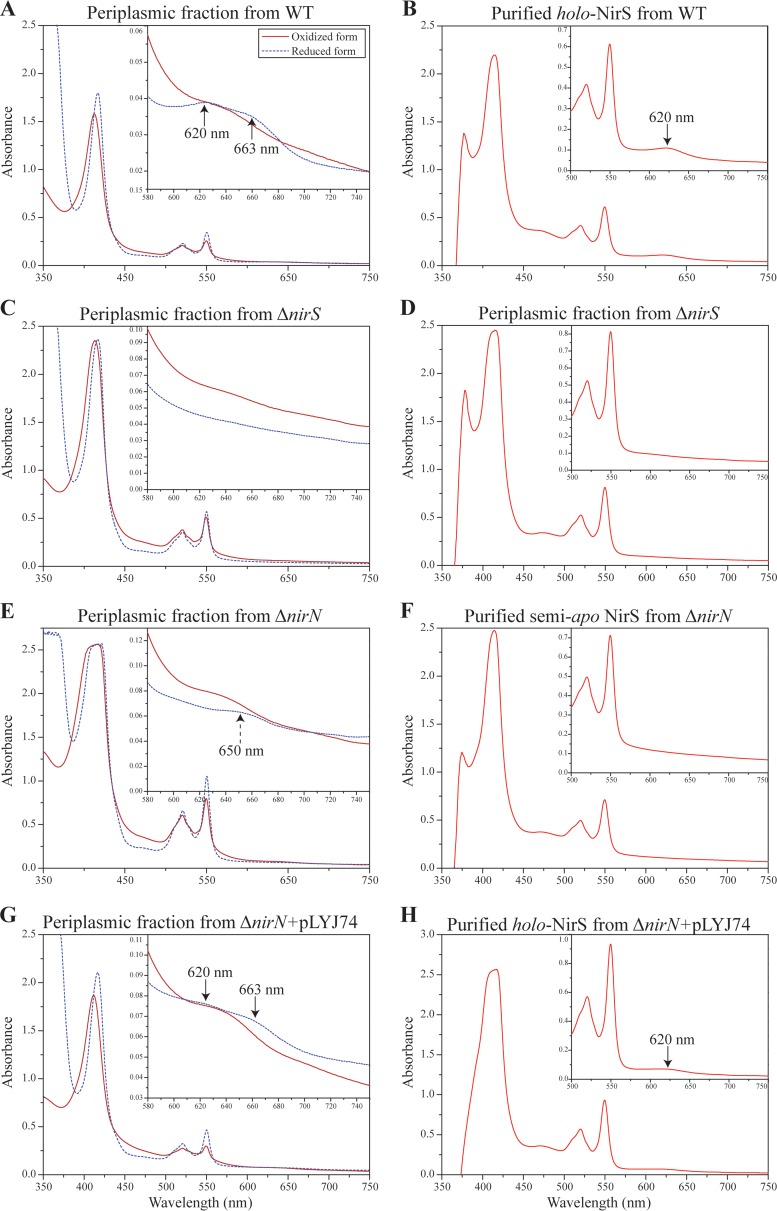
Fig 5.
Spectral analysis of cytochrome cd1 from WT, ΔnirS mutant, ΔnirN mutant, and ΔnirN mutant plus pLYJ74. (A) UV-visible absorption spectra of oxidized and sodium dithionite reduced periplasmic fraction from WT cells showing characteristic spectra (black arrow) for cytochrome cd1. (B) Pyridine ferrohemochrome spectrum of purified holo-cytochrome cd1 (in 50 mM Tris-HCl [pH 7.5]) from WT cells. A characteristic peak for d1 heme at 620 nm was present (black arrow). (C) UV-visible absorption spectra of oxidized and sodium dithionite-reduced periplasmic fractions from the ΔnirS mutant for cytochrome cd1. No peak for d1 heme was found due to the deletion of nirS. (D) Pyridine ferrohemochrome spectrum of the periplasmic fraction (in 50 mM Tris-HCl [pH 7.5]) from the ΔnirS mutant. To avoid the effects from other heme-containing proteins, the periplasmic fraction was purified by DEAE chromatography before analysis. It lacked the characteristic d1 heme absorption peak at 620 nm. (E) UV-visible absorption spectra of oxidized and sodium dithionite-reduced periplasmic fraction from the ΔnirN mutant for cytochrome cd1. The absorption peaks for d1 heme at 620 nm and 663 nm in a reduced form were missing, and instead a new 650-nm shoulder was observed (black dashed arrow). (F) Pyridine ferrohemochrome spectrum of purified semi-apo cytochrome cd1 (in 50 mM Tris-HCl, pH 7.5) from the ΔnirN mutant. No characteristic peak for d1 heme was found. (G) UV-visible absorption spectra of the oxidized and sodium dithionite-reduced periplasmic fractions from the ΔnirN mutant with pLYJ74, which carries nirN. The peaks characteristic for d1 heme (643 nm in oxidized form and 663 and 620 nm in reduced form) were restored (black arrow). (H) Pyridine ferrohemochrome spectrum of purified holo-cytochrome cd1 (in 50 mM Tris-HCl [pH 7.5]) from the ΔnirN mutant with pLYJ74 with plasmid-borne nirN. A characteristic peak for d1 heme was observed (black arrow). The results shown come from one data set representative of two independent experiments.