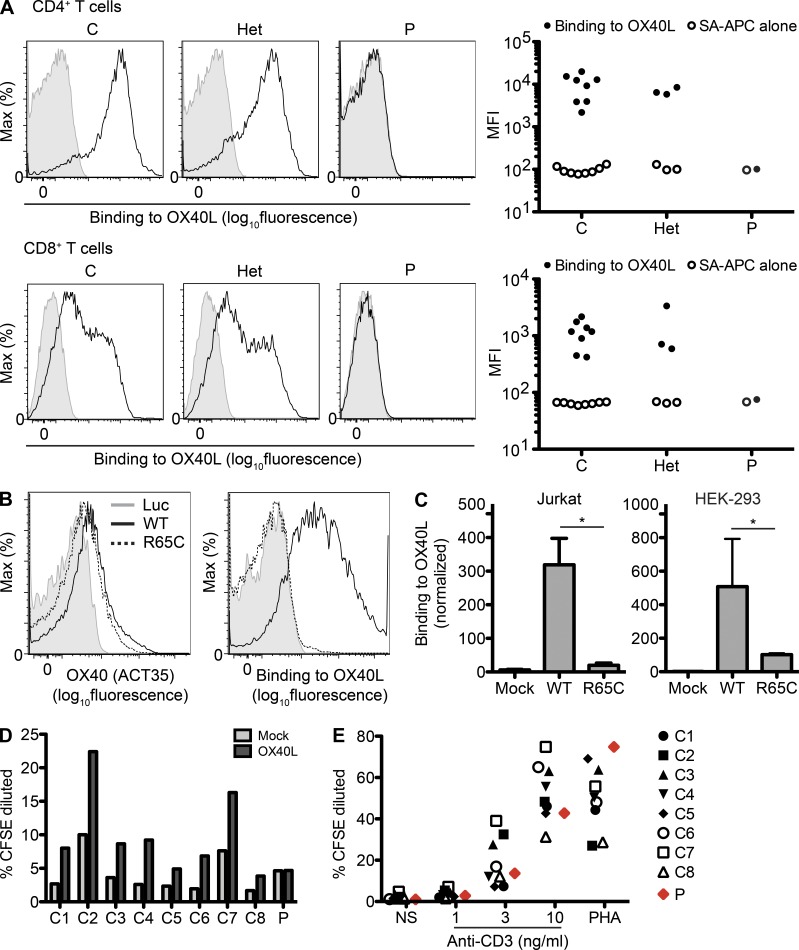
Figure 4.
R65C is a loss-of-function mutation. (A) PHA-activated T cell blasts from healthy controls (C), R65C heterozygous family members (Het), or the patient (P) were incubated with biotinylated recombinant soluble OX40L. Unbound OX40L molecules were washed out, and the levels of cell-bound OX40L were measured by flow cytometry with allophycocyanin-labeled streptavidin (SA-APC). Representative histograms and MFIs (mean fluorescence intensities) of CD3+CD4+ cells (CD4+ T cells) and CD3+CD8+ cells (CD8+ T cells) in three independent experiments are shown. (B) PHA-activated T cell blasts from the patient were transduced with bicistronic lentiviral vectors encoding luciferase (Luc), OX40-WT, or OX40-R65C, together with IRES-RFP. Cell surface OX40 levels measured with ACT35 and binding to OX40L measured with biotinylated recombinant soluble OX40L are shown for CD3+CD4+RFP+ cells. One result representative of two independent experiments is shown. (C) OX40L binding to Jurkat or HEK-293 cells transduced with bicistronic retroviruses with an empty vector (Mock) or encoding OX40-WT or OX40-R65C, together with IRES-GFP, was assessed as in A. The MFIs of OX40L binding for GFP+ cells, normalized with respect to those for isotype controls, are shown. The mean of three independent experiments is shown. Error bars indicate the SEM. *, P < 0.05. (D) CFSE-labeled PBMCs from healthy controls (C1–C8) and the patient (P) were incubated for 3 d with 1 ng/ml of plate-bound anti-CD3 antibody and Vero cells infected with retroviruses either with an empty vector (Vero-Mock) or encoding OX40L (Vero-OX40L). Percentages of CFSE-diluted cells in the CD3+CD4+ population are plotted. One result representative of two independent experiments is shown. (E) CFSE-labeled PBMCs from healthy controls (C1–C8) and the patient (P) were incubated for 3 d with the indicated concentrations of plate-bound anti-CD3 antibody or PHA. Percentages of CFSE-diluted cells in the CD3+CD4+ population are plotted. One result representative of two independent experiments is shown.