Figure 5. Structural rearrangements due to the E78D/E81D mutations.
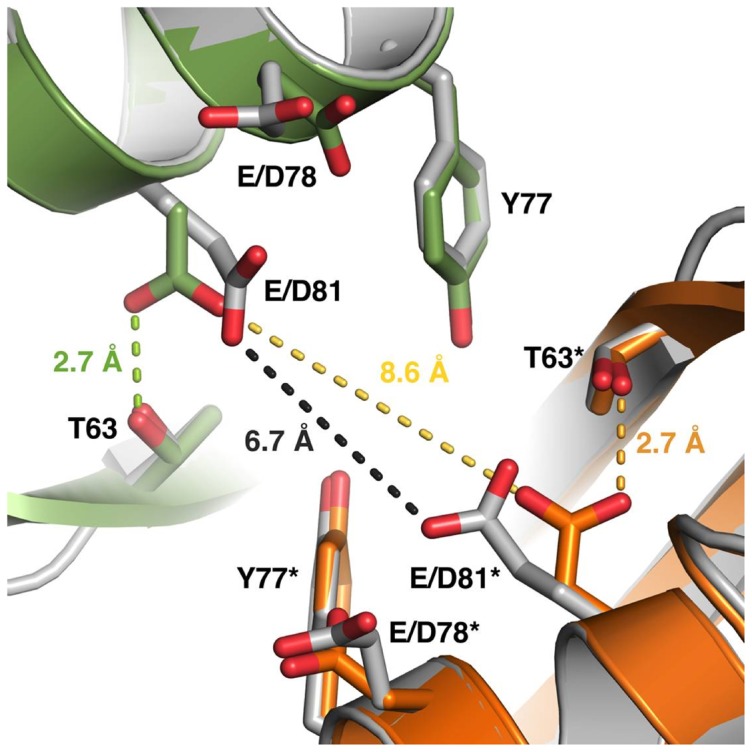
The side chains of the E/D78 and E/D81 residues in the wild-type and the mutated HbpS point towards the corresponding side chains from a neighboring monomer in the octameric assembly. The distance of the two E81 side chains in the wild-type protein (gray) is 6.7 Å, which would allow for iron coordination. Other nearby residues include Y77 and T63. In the mutated protein, this distance has increased to 8.6 Å. Furthermore, the side chain of D81 has turned to form a hydrogen bond with T63. The two monomers in the mutant HbpS are colored green and orange. The residues discussed are labeled, and the asterisk denotes the residues from the symmetry-related molecule in the crystal. The distances between the corresponding E/D81 residues as well as the hydrogen bond between D81 and T63 are marked with dotted lines.
