Abstract
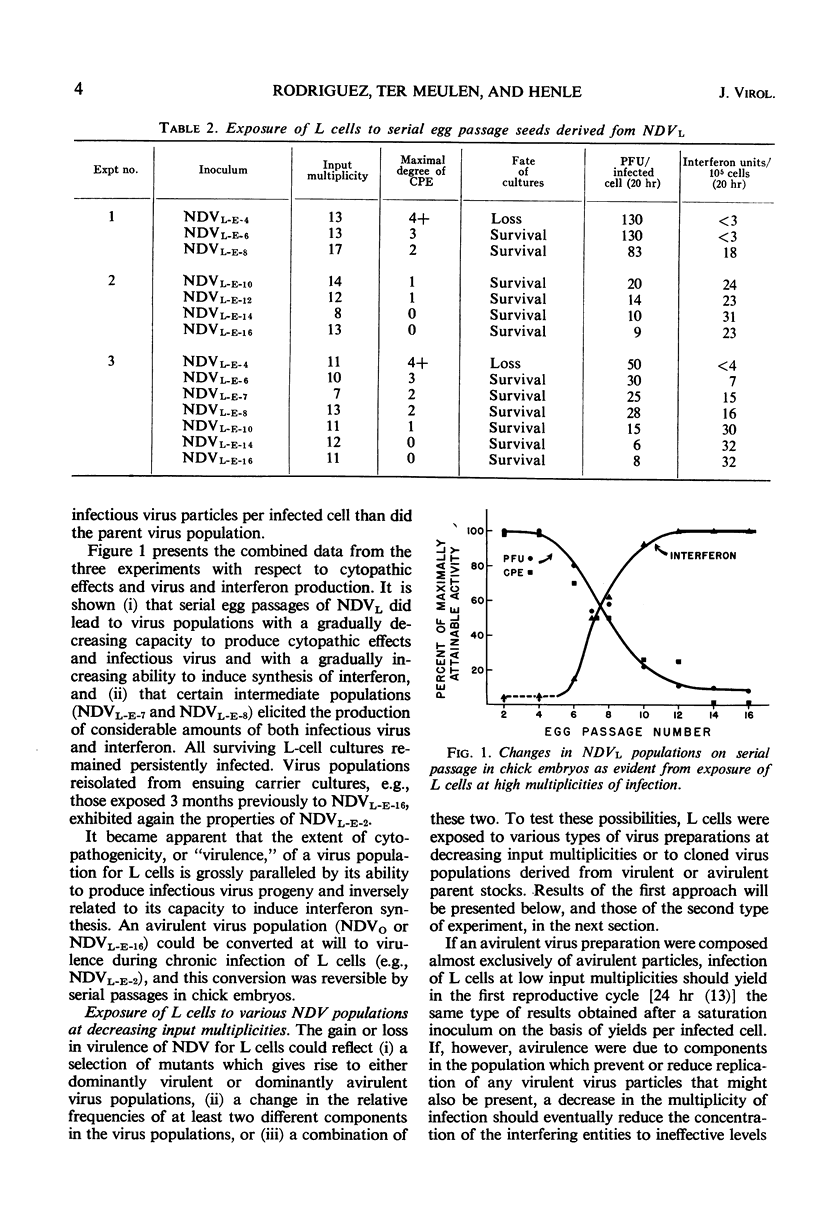
Populations of the Victoria strain of Newcastle disease virus (NDV), reisolated from persistently infected L-cell cultures and passed twice in the embryonated hen's egg (NDVL-E-2), were found to differ strikingly from the original, chick embryo-adapted virus (NDVo). After exposure of L cells to NDVo at high multiplicities of infection, all cells became abortively infected; they produced only small aggregates of viral antigen and few, if any, infectious virus particles, but they yielded large amounts of interferon. No cytopathic effects (CPE) were noted, and the cultures survived readily as viral carriers. In contrast, NDVL-E-2 yielded under similar conditions large quantities of viral antigen and infectious virus particles, but no detectable interferon, and the cultures were rapidly destroyed. This change in “virulence” was at least partially reversible by further serial passages of NDVL-E-2 in chick embryos, as was evident from a consecutive decrease in CPE with a concomitant increasingly rapid recovery of the L-cell cultures, gradually diminishing yields of infectious viral progeny, and the returning of a capacity to induce interferon synthesis. Thus, NDVL-E-16 resembled NDVo in many aspects, except for a less striking reduction in its ability to replicate in L cells. Although a selection of viral variants under the given sets of conditions has not been entirely excluded, the establishment of “avirulence” appears to be largely explained by a gradual accumulation of noninfectious, interferon-inducing components in the course of serial passages in the embryonated hen's egg, and the acquisition of “virulence” by a loss of these components. The evidence is as follows. (i) By a step-wise decrease in the dose of virus and restriction of the analyses to the first infectious cycle, a multiplicity of infection was ultimately reached for all “avirulent” populations at which infected cells produced normal yields of infectious viral progeny; i.e., the interferon-inducing components were diluted to noneffective levels. The lowest multiplicity which resulted in a measurable reduction in infectious virus replication was also the last one to induce detectable interferon synthesis. (ii) All viral clones derived from “avirulent” populations behaved like NDVL-E-2 rather than like the parent viral suspensions, except that some of them elicited small amounts of interferon in L cells. The interferon-inducing components were reduced or lost in the cloning procedures. The nature of the interferon-inducing components has not been established. These components, which were neutralized by rabbit sera against “virulent” NDVL-E-2 populations, may represent largely inactive or incomplete virus particles; however, the infectious virus-hemagglutinin ratios of “avirulent” populations were mostly of an order similar to those of “virulent” populations. The interferon-inducing components aborted the infectious process in cells simultaneously invaded by infectious virus particles. The implications of these findings are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DEINHARDT F., BERGS V. V., HENLE G., HENLE W. Studies on persistent infections of tissue cultures. III. Some quantitative aspects of host cell-virus interactions. J Exp Med. 1958 Oct 1;108(4):573–589. doi: 10.1084/jem.108.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND L. C., MOORE A. E. Changes in activity of Newcastle disease virus after adaption to Ehrlich ascites tissue culture. J Immunol. 1956 Aug;77(2):81–86. [PubMed] [Google Scholar]
- DRAKE J. W., LAY P. A. Host-controlled variation in NDV. Virology. 1962 May;17:56–64. doi: 10.1016/0042-6822(62)90081-8. [DOI] [PubMed] [Google Scholar]
- ENDERS J. F. A consideration of the mechanisms of resistance to viral infection based on recent studies of the agents of measles and poliomyelitis. Trans Stud Coll Physicians Phila. 1960 Oct;28:68–79. [PubMed] [Google Scholar]
- GLASGOW L. A., HABEL K. The role of interferon in vaccinia virus infection of mouse embryo tissue culture. J Exp Med. 1962 Mar 1;115:503–512. doi: 10.1084/jem.115.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANOFF A. Induction of Newcastle disease virus mutants with nitrous acid. Virology. 1961 Apr;13:402–408. doi: 10.1016/0042-6822(61)90270-7. [DOI] [PubMed] [Google Scholar]
- GRANOFF A. Studies on mixed infection with Newcastle disease virus. I. Isolation of Newcastle disease virus mutants and tests for genetic recombination between them. Virology. 1959 Dec;9:636–648. doi: 10.1016/0042-6822(59)90154-0. [DOI] [PubMed] [Google Scholar]
- HENLE G., DEINHARDT F., BERGS V. V., HENLE W. Studies on persistent infections of tissue cultures. I. General aspects of the system. J Exp Med. 1958 Oct 1;108(4):537–560. doi: 10.1084/jem.108.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENLE W., HENLE G., DEINHARDT F., BERGS V. V. Studies on persistent infections of tissue cultures. IV. Evidence for the production of an interferon in MCN cells by myxoviruses. J Exp Med. 1959 Oct 1;110:525–541. doi: 10.1084/jem.110.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISAACS A. INTERFERON. Adv Virus Res. 1963;10:1–38. [PubMed] [Google Scholar]
- MASON E. J., KAUFMAN N. The persistent production of small quantities of infectious Newcastle disease virus in grossly unaltered L and U12 strain cells. J Immunol. 1961 Apr;86:413–420. [PubMed] [Google Scholar]
- RODRIGUEZ J. E., HENLE W. STUDIES ON PERSISTENT INFECTIONS OF TISSUE CULTURES. V. THE INITIAL STAGES OF INFECTION OF L(MCN) CELLS BY NEWCASTLE DISEASE VIRUS. J Exp Med. 1964 Jan 1;119:895–921. doi: 10.1084/jem.119.6.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUIZ-GOMEZ J., ISAACS A. Interferon production by different viruses. Virology. 1963 Jan;19:8–12. doi: 10.1016/0042-6822(63)90018-7. [DOI] [PubMed] [Google Scholar]
- THIRY L. SOME PROPERTIES OF CHEMICALLY INDUCED SMALL-PLAQUE MUTANTS OF NEWCASTLE DISEASE VIRUS. Virology. 1964 Oct;24:146–154. doi: 10.1016/0042-6822(64)90097-2. [DOI] [PubMed] [Google Scholar]
- WAGNER R. R., LEVEY A. H., SNYDER R. M., RATCLIFF G. A., Jr, HYATT D. F. BIOLOGIC PROPERTIES OF TWO PLAQUE VARIANTS OF VESICULAR STOMATITIS VIRUS (INDIANA SEROTYPE). J Immunol. 1963 Jul;91:112–122. [PubMed] [Google Scholar]


