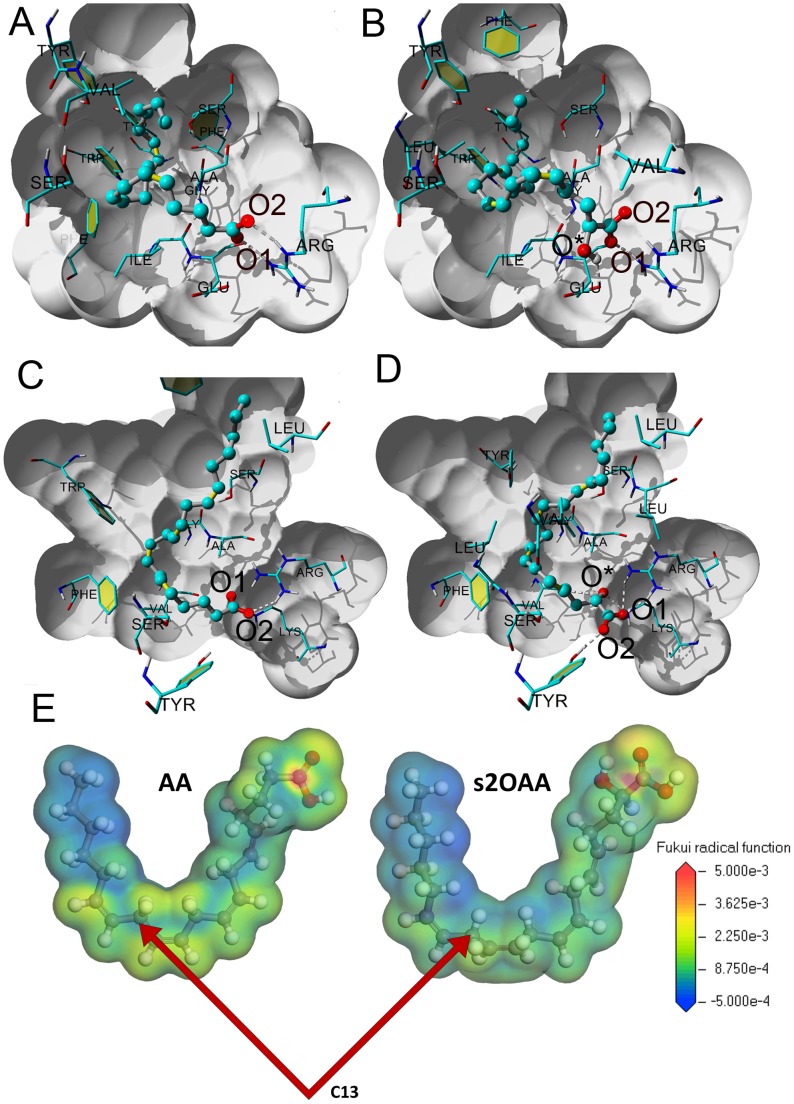
Figure 1. Computational simulations based on molecular docking.
A. AA in the COX1 binding site. B. S2OAA in the COX1 binding site. The two carboxyl oxygens (O1 and O2) of AA establish hydrogen bonds with Arg 120 and they have close hydrophobic contacts with Phe 205, Val 344 and Tyr 348. The orientation of S2OAA is very similar to that of AA, with O1 and O2 occupying the same positions in both. The hydroxyl oxygen (O*) of S2OAA is hydrogen bound to Glu 524, although this favorable interaction is counterbalanced by a distortion of the carbon backbone. To facilitate visual inspection of the interactions, the binding site is shaded in grey, the fatty acids are represented by sticks and balls, and only amino acids closer than 3 Å are shown. C. AA in the COX2 binding site. D. S2OAA in the COX2 binding site. The carboxylate group of AA is coordinated with Arg 2120 by one hydrogen bond, whereas R2OAA possesses five hydrogen bonds. The O* oxygen occupies the position of O1 of AA and in an analogous manner, O1 of S2OAA occupies the position of the AA O2. Finally, O2 of substituted arachidonic acid is free to hydrogen bond to Tyr 2355. The binding site is shaded in grey, fatty acids are represented by sticks and balls, and only amino acids closer than 3 Å are shown. E. The Fukui function f0(r) is color-mapped onto the electron density isosurface with equal isovalues.