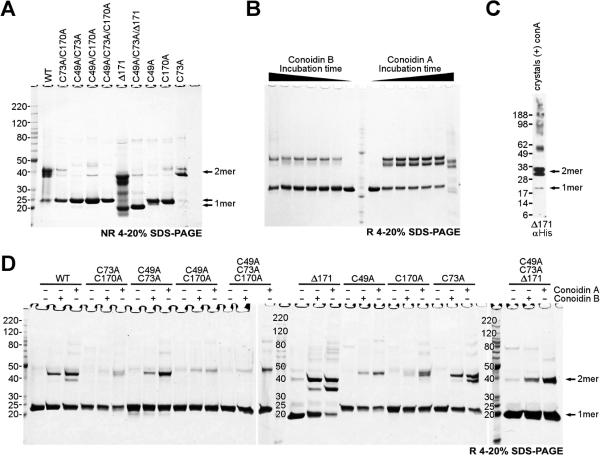
Figure 3. SDS-PAGE analysis shows that both conoidin A and conoidin B react with AcePrx-1.
AcePrx-1 was purified under reducing conditions and desalted immediately prior to sample preparation. A. Wild type and mutant AcePrx-1 under non-reducing conditions. Most of the wild type protein is a disulfide-linked dimer. Neither a C73A mutation nor a deletion of C-terminal residues 172-196 affect dimerization. Mutation of Cys49 or Cys170 renders the protein predominantly monomeric.
B. Time course assay of wild type AcePrx-1 reactivity with conoidin A and conoidin B. The time points are 0 h, 0.5 h, 1 h, 3 h, 6 h, 24 h and 96 h. The samples were boiled in Laemmli buffer to quench reactivity, and then run under reducing conditions. Dimeric species appear within only 0.5 h and remain stable. After 96 h, appreciable degradation was observed in these samples in the presence of conoidin A but not of conoidin B over the same duration. Faint bands corresponding to higher-order oligomers were observed after 96 h.
C. Reducing gel from dissolved crystals of the AcePrx-1(Δ171)/conoidin A complex. Conidin A covalently crosslinks AcePrx-1 into dimers. Traces of monomer and higher order oligomers are visible.
D. Reducing gel showing that conoidin A and conoidin B cause covalent (or non-covalent SDS-resistant) oligomerization of wild type and mutant AcePrx-1 after incubation for 24 h. The appearance of higher-order oligomer bands in the C49A/C73A/C170A triple mutant suggest that side chains other than cysteine can react with conoidin A nonspecifically and lead to crosslinking of AcePrx-1. See also Table S1.