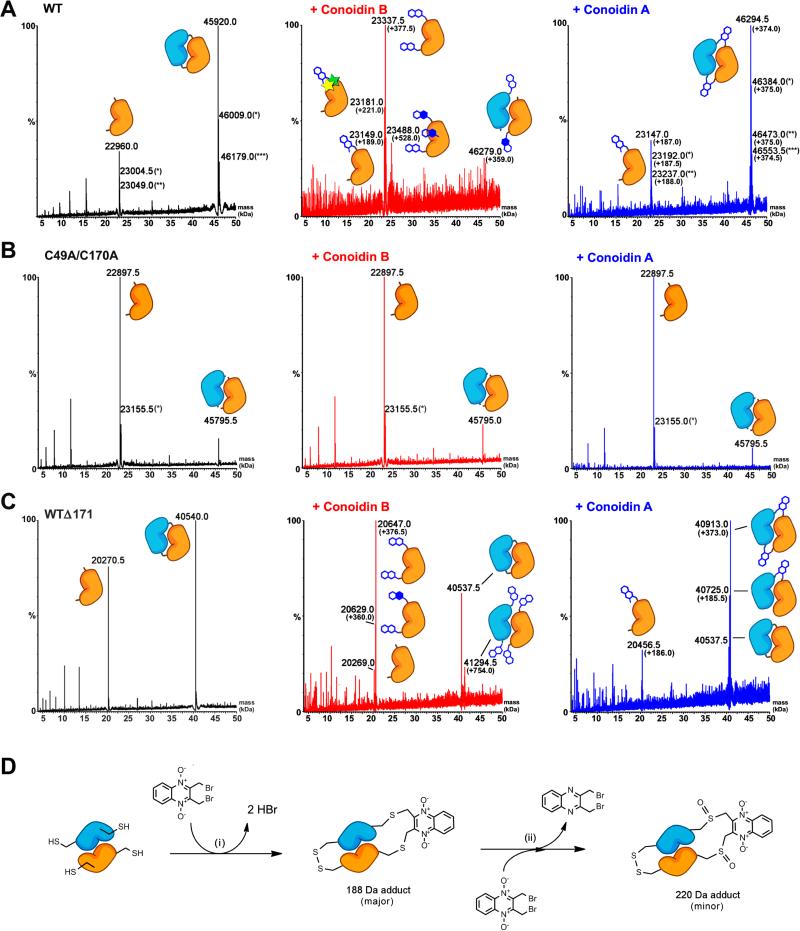
Figure 4. Conoidin A and conoidin B covalently modify AcePrx-1 by alkylation or crosslinking.
Liquid chromatography and electrospray ionization mass spectra (LC-ESI-MS) of wild type and mutant AcePrx-1 are shown without pretreatment (left column), after treatment with conoidin B (middle column) and after treatment with conoidin A (right column). The chemical composition of any adducts is shown schematically next to each peak in the spectra. Quinoxaline dioxide (QDO) adducts are represented as two open hexagons. Filled hexagons indicate deoxygenation to the mono-oxide. Each star represents addition of a single oxygen to the protein.
A. Spectra for wild type AcePrx-1 after a 6-h treatment. Wild type AcePrx-1 is predominately dimeric with a mass of 45,920 Da. Additional peaks represent the monomer (22,960 Da) and various other protonation states, indicated by asterisks. Conoidin B treatment results in alkylation of up to three sites on the monomer, while conoidin A treatment results in alkylation with a single QDO adduct, or crosslinking by two QDO adducts.
B. The C49A/C170A double mutant does not react with conoidin A or conoidin B, even after treatment for 24 h (shown here), suggesting that the catalytic cysteine residues are the specific sites of modification in AcePrx-1.
C. A C-terminal deletion mutant with residues 172-196 missing produces a very similar set of adducts to wild type AcePrx-1 (24-h conoidin A/B treatment shown here).
D. Proposed mechanism of conoidin A inhibition of AcePrx-1. A peroxiredoxin dimer (orange and blue), drawn schematically in its fully folded conformation undergoes successive SN2 reactions with a single molecule of conoidin A, (i), to generate a 188-Da crosslinked adduct. An additional minor product is formed by oxidation of the thioether moieties by the N-oxide on conoidin A, (ii), which produces the 220 Da covalent adduct observed in our mass spectrometry experiments. See also Figure S1.