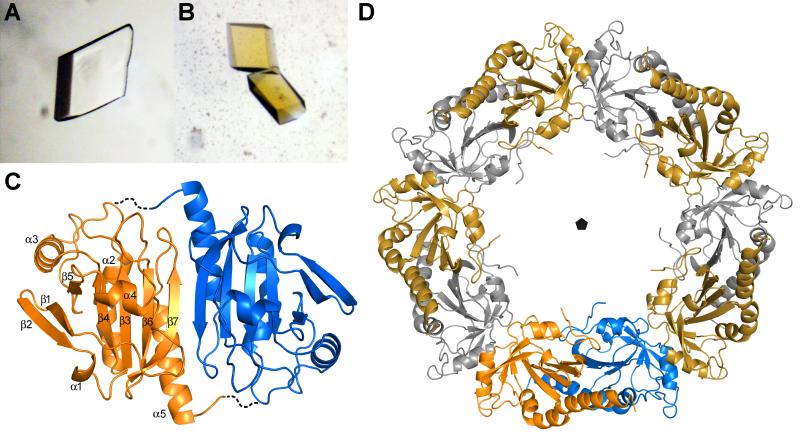
Figure 5. Crystal structure of AcePrx-1.
A. Crystals of AcePrx-1 are colorless in the absence of conoidin A.
B. AcePrx-1 crystals grown in the presence of conoidin A are yellow, suggesting that conoidin A binds to the enzyme.
C. AcePrx-1 adopts a fold similar to other peroxiredoxins. The most similar structure is human Prx-4 (PDB code 3TJB). AcePrx-1 forms dimers principally via inter-strand contacts to form an eight-stranded β-sheet. Dashed lines connect the active site cysteines across the dimer interface.
D. Five AcePrx-1 dimers assemble into a decamer in solution and in the crystal structure. See also Figure S2.