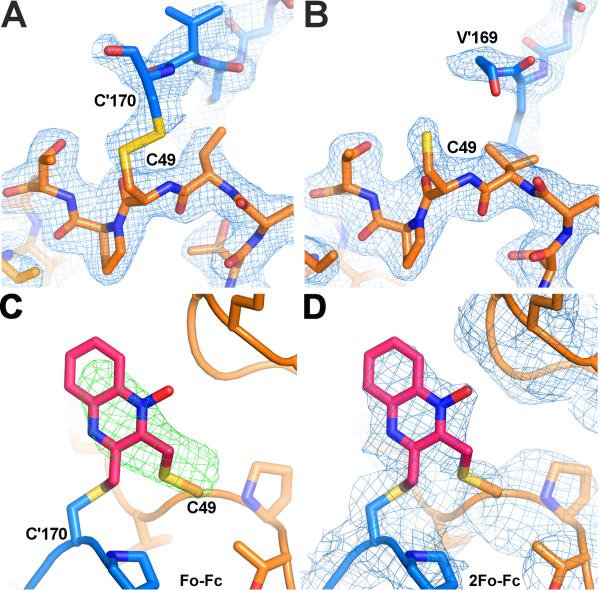
Figure 6. The active site of AcePrx-1 in the presence and absence of conoidin A.
A. The structure of AcePrx-1 shows that the enzyme is in the locally unfolded conformation, with disulfide bonds between the peroxidatic and resolving cysteine residues (Cys49 and Cys170, respectively) visible in two of the ten active sites in the decameric asymmetric unit (B, C). Subunits A (blue) and B (orange) are shown.
B. In the remaining subunits (A, D-J), Cys170 is disordered and electron density is lacking for the disulfide bond and helix α6 (subunit E is in orange).
C. Positive electron density is present in Fo - Fc maps (green) near the active site cysteine residues of AcePrx-1 Δ171 co-crystallized with conoidin A. A quinoxaline monoxide (QMO) adduct was modeled into the density and refined. The view is rotated horizontally ~90°, then vertically 180° relative to the view in panel A.
D. 2Fo - Fc map (blue) after refinement with the QMO adduct. See also Figure S3.