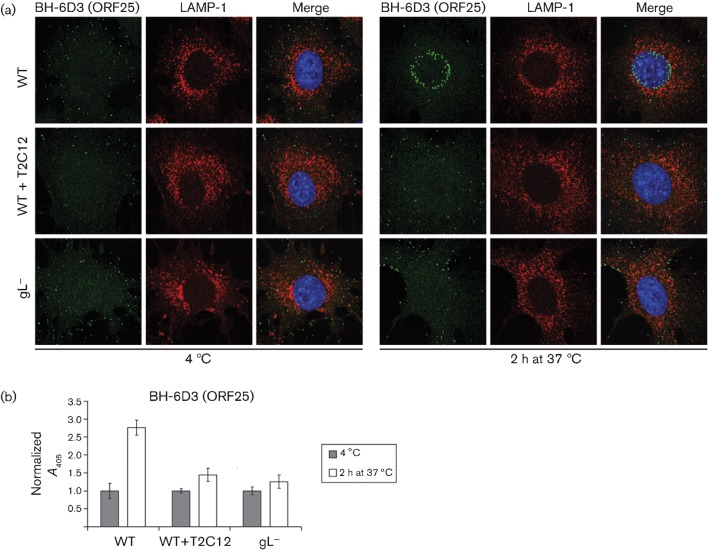
Fig. 3.
Capsid migration of T2C12-neutralized and gL− virions. (a) WT MuHV-4 virions (3 p.f.u. per cell) were left untreated or pre-incubated (2 h, 37 °C) with mAb T2C12 (anti-gH–gL, IgG2a, 400 µg ml−1). WT (3 p.f.u. per cell) and gL− (50 p.f.u. per cell to give equivalent binding) virions were then bound to NMuMG cells for 2 h at 4 °C. The cells were washed with PBS and either fixed immediately or first incubated (2 h, 37 °C) to allow virion endocytosis. All cells were then stained for the ORF25 virion capsid component with mAb BH-6D3 (IgG1, green), for the late endosomal marker LAMP-1 (red), and with DAPI (blue). Equivalent data were obtained in a repeat experiment. (b) Cells were exposed to WT virions with or without T2C12 neutralization or to gL− virions as in (a), but antibody binding was detected with an alkaline phosphatase-conjugated IgG1-specific secondary antibody, p-nitrophenylphosphate substrate and A405. For each condition, the A405 was normalized to the value obtained at 4 °C. The bars show mean±sem values from six wells. After incubation at 37 °C, the non-neutralized WT signal was significantly higher than that of gL− or T2C12-treated WT virions (P<0.0005 by Student’s t-test). Equivalent data were obtained in two further experiments.