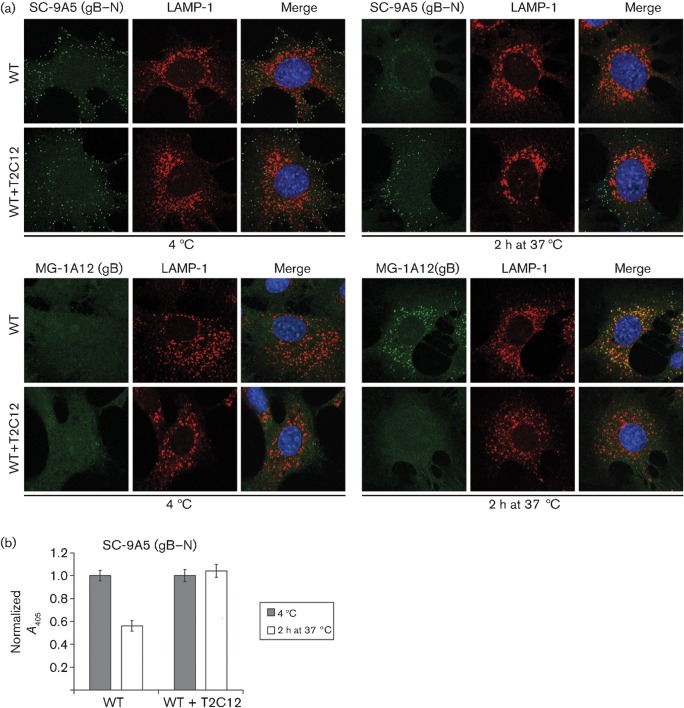
Fig. 6.
gH–gL-directed neutralization prevents conformation changes in gB. (a) WT MuHV-4 virions (3 p.f.u. per cell) were left untreated or pre-incubated (2 h, 37 °C) with mAb T2C12 (IgG2a, 400 µg ml−1), then bound to NMuMG cells (2 h, 4 °C). The cells were washed with PBS and either fixed immediately or first further incubated (2 h, 37 °C) to allow virion endocytosis, then stained with mAb SC-9A5 (IgG3), which is specific for pre-fusion gB, and with mAb MG-1A12 (IgG2a), which is specific for post-fusion gB (Glauser et al., 2011). SC-9A5 was detected with an Alexa Fluor 488-conjugated goat anti-mouse IgG3 pAb; MG-1A12 was conjugated directly to Alexa Fluor 488 (both green). The cells were also stained for LAMP-1 (red) and with DAPI (blue). (b) NMuMG cells were exposed to untreated or T2C12-neutralized WT virions as in (a), then stained for pre-fusion gB with mAb SC-9A5. Antibody binding was detected with alkaline phosphatase-conjugated IgG3-specific secondary antibody, p-nitrophenylphosphate substrate and A405. For each condition, the A405 was normalized to the value obtained at 4 °C. The bars show mean±sem values from six wells. The non-neutralized WT signal was reduced significantly after incubation at 37 °C (P<10−4 by Student’s t-test), whereas the neutralized WT signal was not changed significantly (P>0.58). Equivalent data were obtained in a repeat experiment.