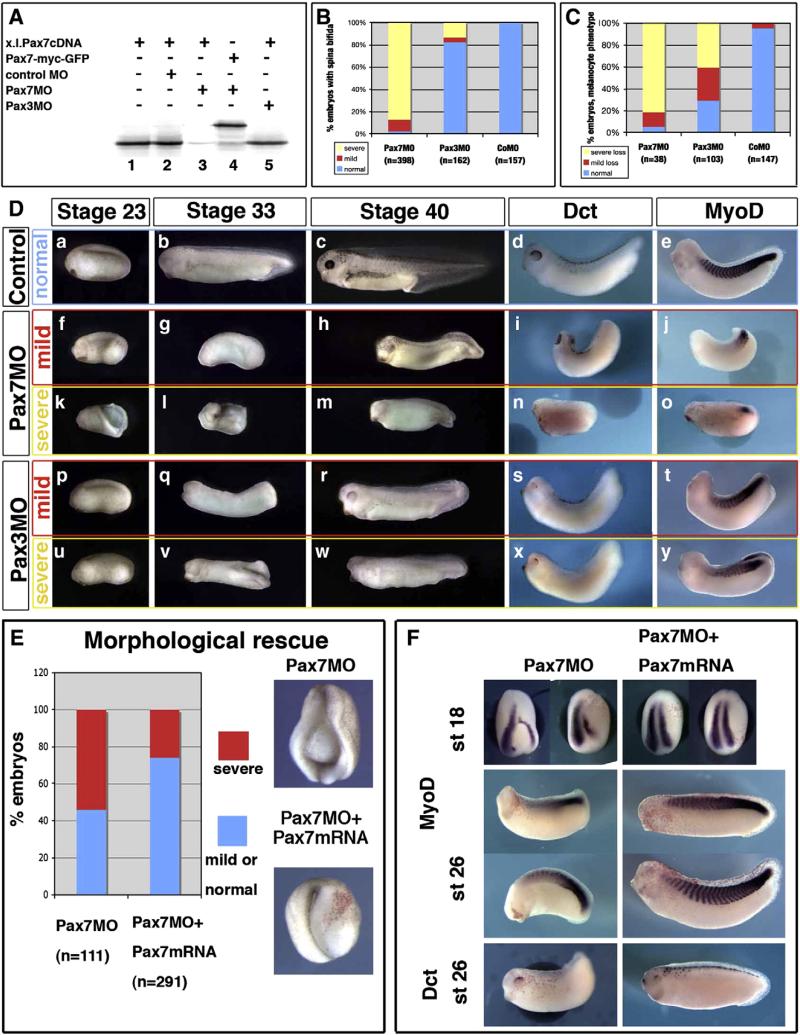
Fig. 6.
Pax7 morphants are affected in earlier stages than Pax3 morphants, although both display mesoderm and neural crest defects. (A) Pax7MO blocks in vitro translation of pax7 (lane 3) but neither that of a pax7-gfp fusion lacking the MO-binding sequence (lane 4), nor of pax3 (5). (B) Pax7 morphants display early and severe elongation defects shortly after gastrulation (yellow), while a few of them exhibit milder phenotypes allowing us to follow their development further (red). In contrast, Pax3 morphants are rather normal until the end of neurulation except for posterior spina bifida in the more severely affected ones (yellow). (C) Melanocyte development was analyzed in later stage embryos, among the mild phenotype for Pax7 morphants, and in Pax3 morphants. Severe loss refers to the lack of dct positive or pigmented cells, while “mild loss” refers to decreased melanocyte number associated to lack of melanocyte migration. (D) Sibling control embryos (a–e), Pax7 morphants with mild (f–j) or severe (k–o) phenotype, Pax3 morphants with mild (p–t) or severe (u–y) phenotype were analyzed at the end of neurulation (stage 23), tailbud stage 33 or swimming tadpole stage 45, and stained for dct and myod at stage 33. They display prominent head, brain, mesoderm and melanocytes defects. (E)The Pax7MO phenotype is rescued by co-injections with Pax7-myc mRNA, insensitive to the morpholino. See text for details. (F) Both myoD and dct expression are rescued by pax7 mRNA injection into Pax7 morphants (see text for details).