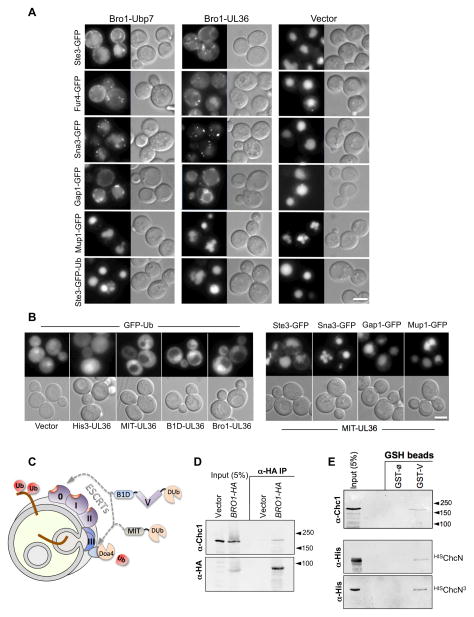
Figure 2. Effect of Bro1-DUb fusion protein on the sorting of MVB cargo.
A. Bro1 fusion proteins containing the catalytic domain of either Ubp7 (Bro1-Ubp7) or UL36 (Bro1-UL36) were expressed from the copper-inducible CUP1 promoter in wild-type cells, in combination with the indicated GFP-tagged MVB cargo proteins. Shown are DIC and GFP fluorescence images.
B. Sorting of GFP-Ub in wild-type cells or in cells expressing the UL36 fused to His3 (His3-UL36), the N-terminal MIT domain of Vps4 (MIT-UL36), the N-terminal Bro1 domain (B1D-UL36), or full-length Bro1 (Bro1-UL36) (left). Wild-type cells or cells expressing a His3-UL36 fusion protein accumulate some GFP-Ub within vacuoles. Expression of Bro1-UL36 or MIT-UL36 excludes GFP-Ub from vacuoles. MIT-UL36 was also co-expressed in WT cells with the indicated GFP-tagged MVB cargo (right). Bar=5μM
C. Model for how Bro1 might work in two places: early in the process of cargo sorting in conjunction with ESCRT-0, such that the Bro1-DUb fusion proteins deubiquitinate cargo and block subsequent sorting into the MVB pathway; and late in the sorting process in conjunction with ESCRT-III, to recycle Ub from the MVB pathway and thus prevent its accumulation in the vacuole. This latter function can be mimicked by MIT-DUb.
D. Immunopreciptiation of HA-tagged Bro1 from spheroplasts prepared from cells transformed with vector alone or plasmid expressing Bro1-HA. Samples were immunoblotted with monoclonal antibodies against Chc1 (top) or HA (bottom).
E. Pulldown of GST alone (∅) or GST fused to the Bro1 V domain (GST-V). Bead-bound fractions were immunoblotted as was a 5% equivalent of input. Top shows pulldown of Chc1 from yeast lysates. Bottom shows pulldown of a monomeric 6xHis-tagged recombinant N-terminal clathrin β-propeller (HISChcN), or an oligomeric version containing a trimerization domain (HISChcN3).