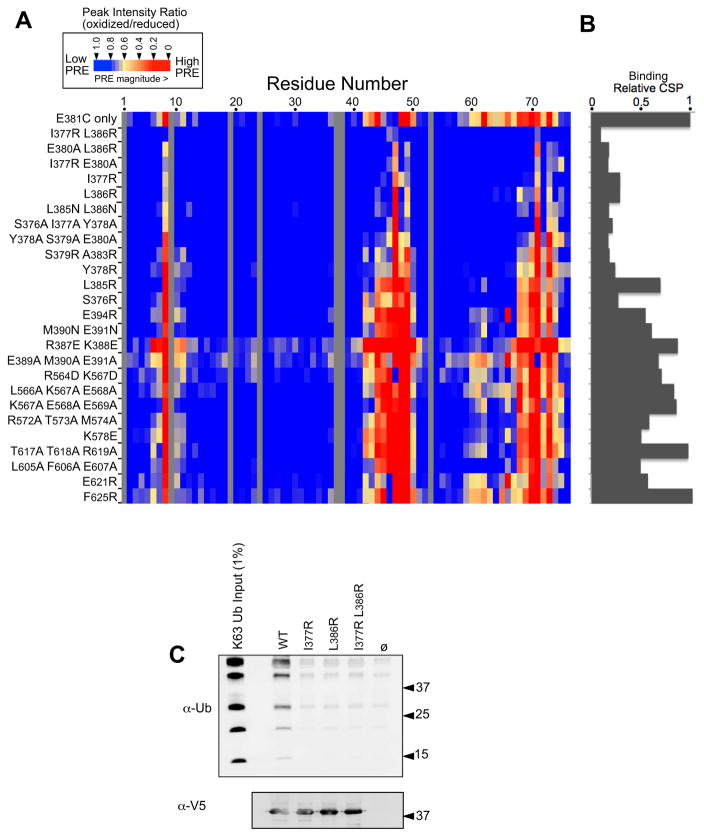
Figure 6. Mutagenesis of the Ub-binding region of Bro1 V domain.
A. The indicated mutations were made in the context of the S. castelli Bro1 domain, which contains a cysteine residue at position 381. These proteins were labeled with MTSL and used in PRE experiments with 15N-Ub at a Brol V:Ub ratio of 3:1. Peak intensity ratios (oxidized vs. reduced spin label) for each Ub residue were calculated from HSQC spectra collected in the absence and presence of 2 mM ascorbate. The degree of PRE effect experienced by each Ub backbone amide is color-coded as indicated.
B. Index of chemical-shift perturbations in 15N-Ub upon binding to mutant Bro1 V domains. CSP index was calculated by summing the (0.2ΔN2 + ΔH2)1/2 values for Ub residues 8,42,44,48,49,69,70 and 71 and setting that value equal to 1 for the WT Bro1 V domain.
C. The WT and mutant recombinant V domains were immobilized on α-V5 polyclonal antibody-coated beads, washed, and incubated with K63-linked polyubiquitin chains. Beads alone or bound to an irrelevant V5-epitoped protein were also included. Beads were washed and immunoblotted with α-V5 or α-Ub monoclonal antibodies.