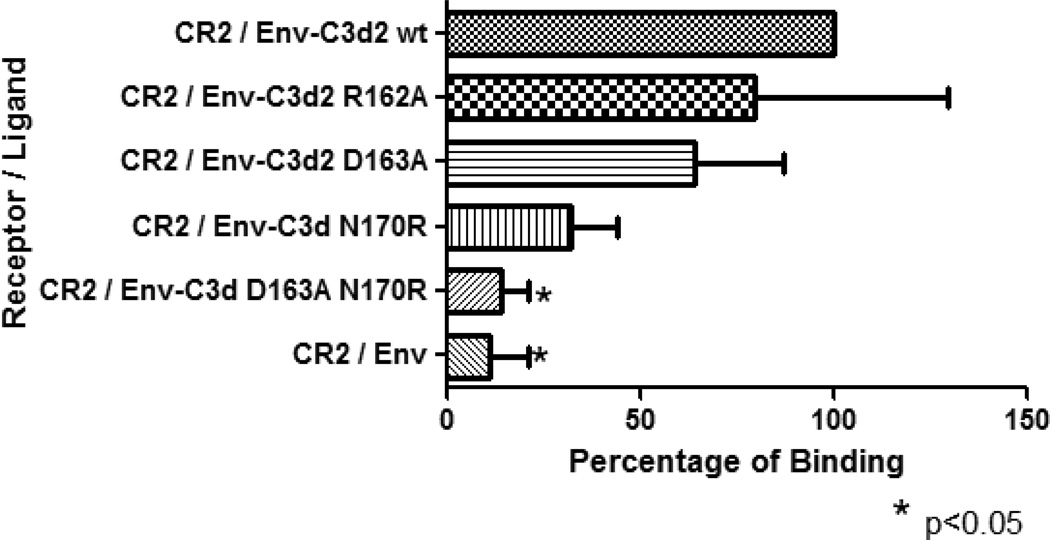
Figure 3. ELISA binding assay.
Envgp120 alone and fused to two copies of wild-type or mutant C3d were assayed for their binding capacity to sCR2. Envgp120- C3d2 wild-type was used as the standard control. Mutation at residue 162 did not alter the binding capacity of Envgp120- C3d2 (R162A) to CR2. Mutations at residues D163A and N170R reduced but not statistically significant the binding of C3d to CR2. Introduction of the double mutation (D163A-N170R) in C3d, synergized and eliminated the binding avidity of C3d for CR2 (p<0.05). As expected, Envgp120 alone showed only background levels of binding to CR2. The experiment was repeated twice in triplicate.