Table 2.
Studies reporting chemotherapy efficacy in sporadic BRCAness
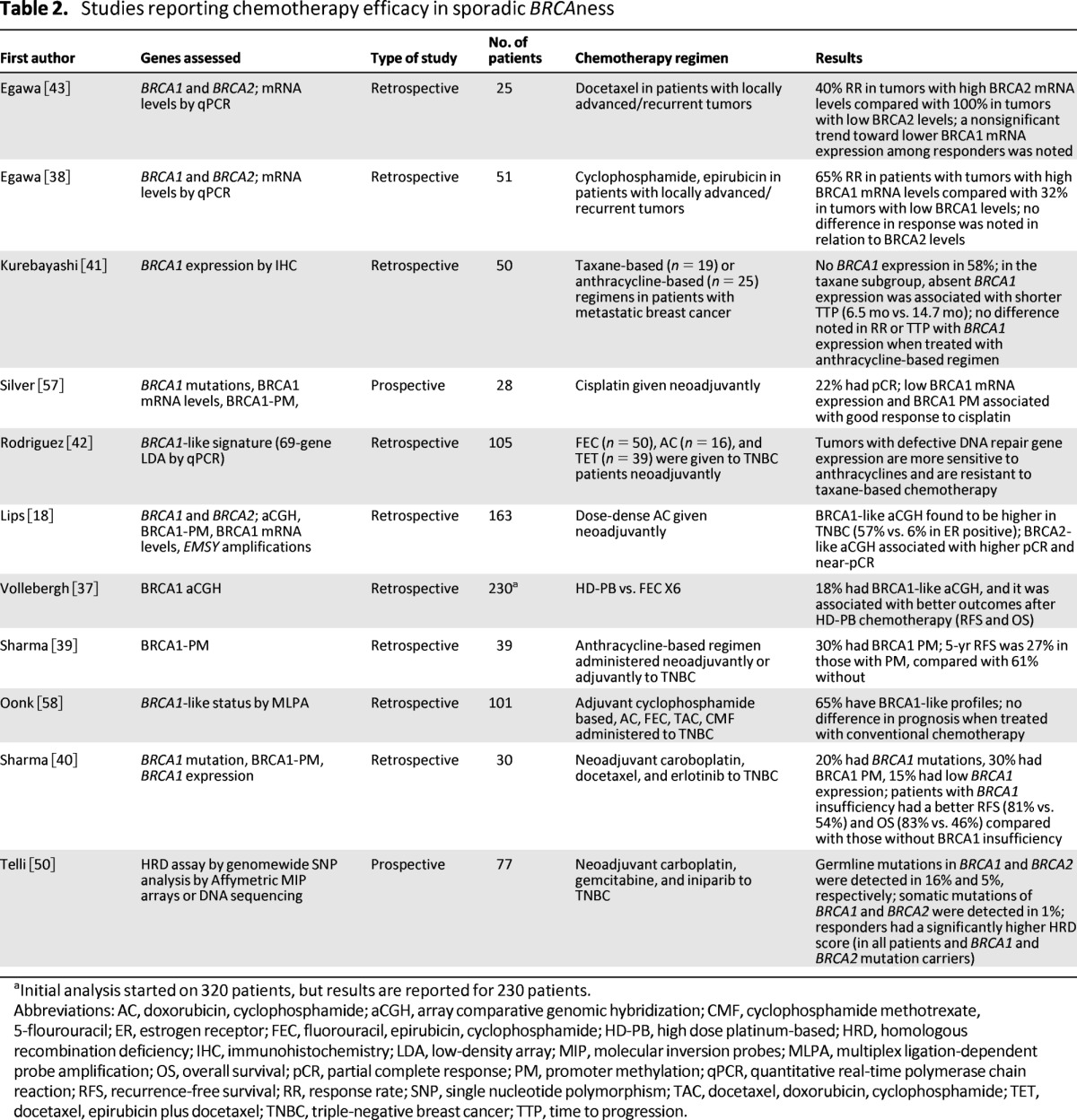
aInitial analysis started on 320 patients, but results are reported for 230 patients.
Abbreviations: AC, doxorubicin, cyclophosphamide; aCGH, array comparative genomic hybridization; CMF, cyclophosphamide methotrexate, 5-flourouracil; ER, estrogen receptor; FEC, fluorouracil, epirubicin, cyclophosphamide; HD-PB, high dose platinum-based; HRD, homologous recombination deficiency; IHC, immunohistochemistry; LDA, low-density array; MIP, molecular inversion probes; MLPA, multiplex ligation-dependent probe amplification; OS, overall survival; pCR, partial complete response; PM, promoter methylation; qPCR, quantitative real-time polymerase chain reaction; RFS, recurrence-free survival; RR, response rate; SNP, single nucleotide polymorphism; TAC, docetaxel, doxorubicin, cyclophosphamide; TET, docetaxel, epirubicin plus docetaxel; TNBC, triple-negative breast cancer; TTP, time to progression.
