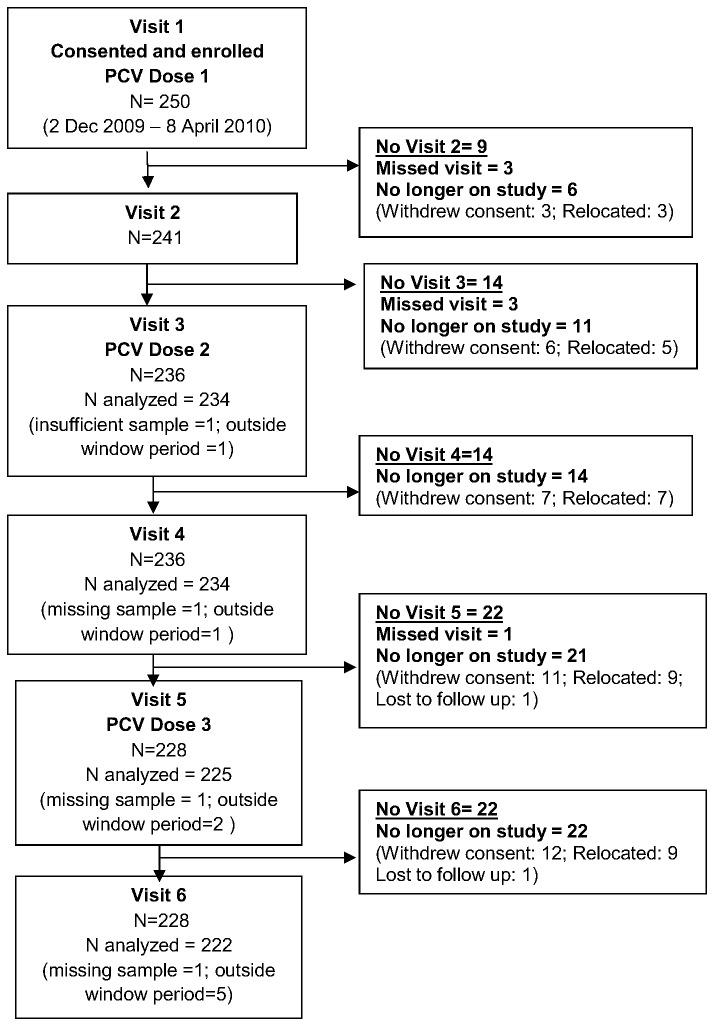
Figure 1. Flow Diagram of study participation.
Diagram indicating number of children enrolled into the study and number excluded or lost to follow-up during the course of the study.
Withdrew consent = participant no longer wished to be part of study cohort and preferred to be vaccinated at local clinic. Relocated=participant moved out of study area and therefore unable to attend study visits. Lost to follow up = study site unable to contact study participant. Outside window period = vaccination occurred outside protocol defined period of 6-12 weeks; 12-24 weeks and 38-42 weeks for the first, second and third doses respectively. PCV= 7-valent Pneumococcal Conjugate Vaccine.