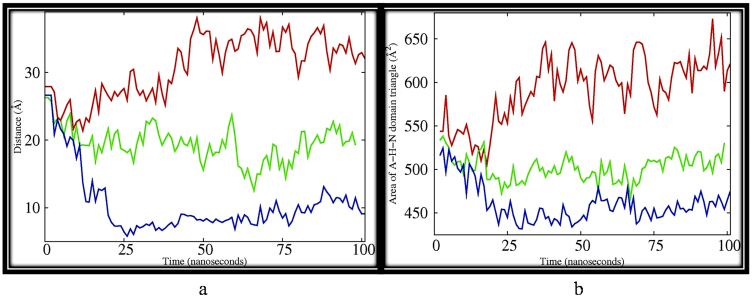
Figure 4.
(a) Inter-atomic distances of backbone atom of VAL 221 of actuator domain and PHE 535 of nucleotide domain for (i) PfATP6 (red), (ii) artemisinin bound PfATP6 (green) and (iii) Fe-artemisinin adduct bound PfATP6 (blue) depicting the two residues coming closer from 25Å to almost 8 Å for most part of the simulation (iii). However smaller variation in the distances were noticed with PfATP6 (red), (26 to 32 Å). For artemisinin bound PfATP6 system (green) (26- 20 Å). (b) Area between the geometric centers of N-, H- and A- domains of PfATP6 system (red), artemisinin–PfATP6 system (green) and Fe-artemisinin adduct PfATP6 system (blue). Areas are calculated from triangles formed by the geometric centers of the three corresponding domains.