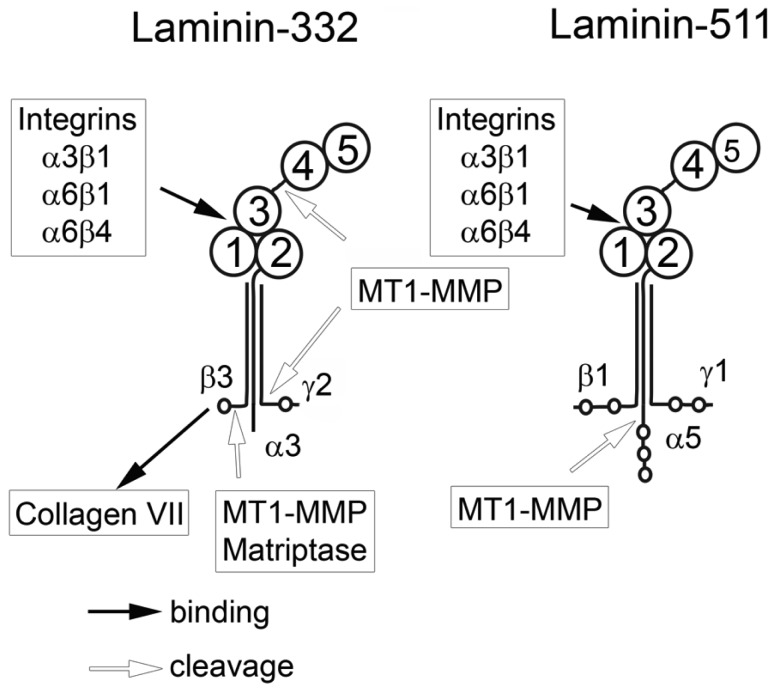
Figure 2.
Structures of the non-processed laminin-332 and laminin-511. A characteristic hallmark of laminins is the α-helical coiled-coil domain consisting of three laminin chains, α3β3γ2 or α5β1γ1 for laminin-332 and laminin-511, respectively. Each chain can be cleaved by several different proteases with drastic effects on its biological functions. Exclusively formed by the C-terminal portion of the α3 chain, the globular (G) domain can be subdivided into five LG-domains, each about 200 amino acids in length. The LG4-5 tandem domain is connected to the LG1-3 domain cluster via connecting sequence. The connecting sequence contains target sites for several proteases. The G-domain contains the putative binding site for the integrins α3β1, α6β1, and α6β4. Secreted laminin-332 undergoes fast processing by proteases which cleave the LG4-5 domain of the α3 chain and the N-terminal domain of the γ2 chain.