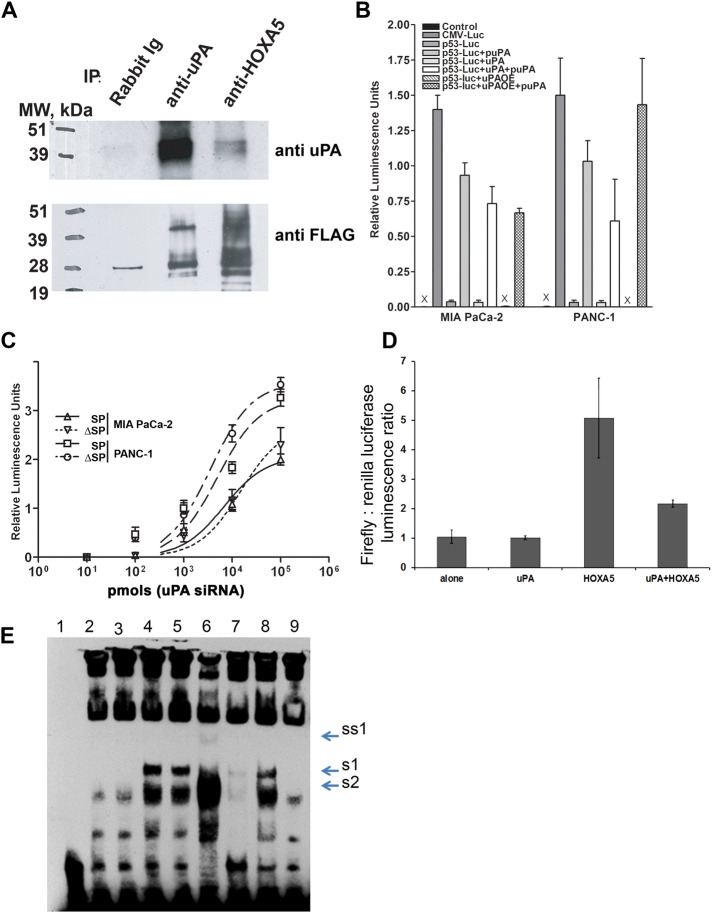
FIGURE 6:
uPA suppresses p53 promoter activity via binding to and attenuation of the Hoxa-5 function. (A) HEK293 cells were transfected with HOXA5-FLAG in pcDNA3.1 and uPA/pcDNA3.1 vector. Two days after transfection, cells were harvested, and nuclear extracts were prepared using the Novagen NucBuster Protein Extraction Kit. uPA and/or HOXA5 were immunoprecipitated using anti-uPA mouse monoclonal Abs (IMTEK, Moscow, Russia) and rabbit polyclonal anti-HOXA5 Abs (Santa Cruz Biotechnology). Immunoprecipitated proteins were subjected to Western blot analysis. Immunoprecipitated and coimmunoprecipitated uPA and/or HOXA5-FLAG were detected using anti-uPA rabbit polyclonal Abs (389; American Diagnostica, Stamford, CT) and mouse monoclonal HRP-conjugated anti-FLAG M2 Abs (Sigma-Aldrich). (B) MIA PaCa-2 and PANC-1 cancer cells were stably transfected with p53 promoter luciferase reporter plasmid. uPA expression was suppressed with puPA or uPA was overexpressed (uPAOE) in these cells. In parallel, cells were incubated with WT-uPA. p53 promoter activity was determined by measurement of luciferase activity as described in Materials and Methods. (C) MIA PaCa-2 and PANC-1 cells were stably transfected with p53-luc plasmid and sorted to obtain SP and ΔSP cells. uPA was suppressed or overexpressed or added to the cells, and luciferase activity was measured as described. (D) HEK293 cells were cotransfected with p53-luc, uPA/pcDNA3.1+ plasmid encoding human WT-uPA, or HOXA5-FLAG/pcDNA3.1 plasmid encoding C-terminus–tagged HOXA5-FLAG. Empty pcDNA3.1 was used as the negative control, and pRL TK plasmid encoding constitutively expressed Renilla luciferase was cotransfected to normalize the data. Luciferase activity was determined using a Promega Dual Luciferase Reporter Assay Kit. (E) Effect of uPA on DNA-binding capacity of HOXA5. HEK293 cells were transfected either with empty pcDNA3.1 (mock transfected) or with HOXA5-FLAG in pcDNA3.1 alone or in combination with uPA/pcDNA3.1 vector. Two days after transfection, cells were harvested. Nuclear extracts were prepared using the NucBuster Protein Extraction Kit. EMSA reactions were performed using biotinylated, double-stranded, p53 promoter-derived Hoxa-5–specific oligonucleotides. 1, No NE; 2, + mock-transfected NE; 3, + uPA-transfected NE; 4, + HOXA5-transfected NE; 5, + HOXA5-transfected NE + BSA; 6, + HOXA5-transfected NE + scuPA (500 ng); 7, + HOXA5-transfected NE + specific “cold” oligo duplex; 8, + HOXA5-transfected NE + scuPA + anti-uPA Abs; 9, + mock-transfected NE + scuPA (500 ng). S1, Probe shift, caused by HOXA5 overexpression; S2, DNA–protein complex, formed in presence of NE from the mock-transfected cells; SS1, probe supershift caused by HOXA5-bound anti-HOXA5 Ab.