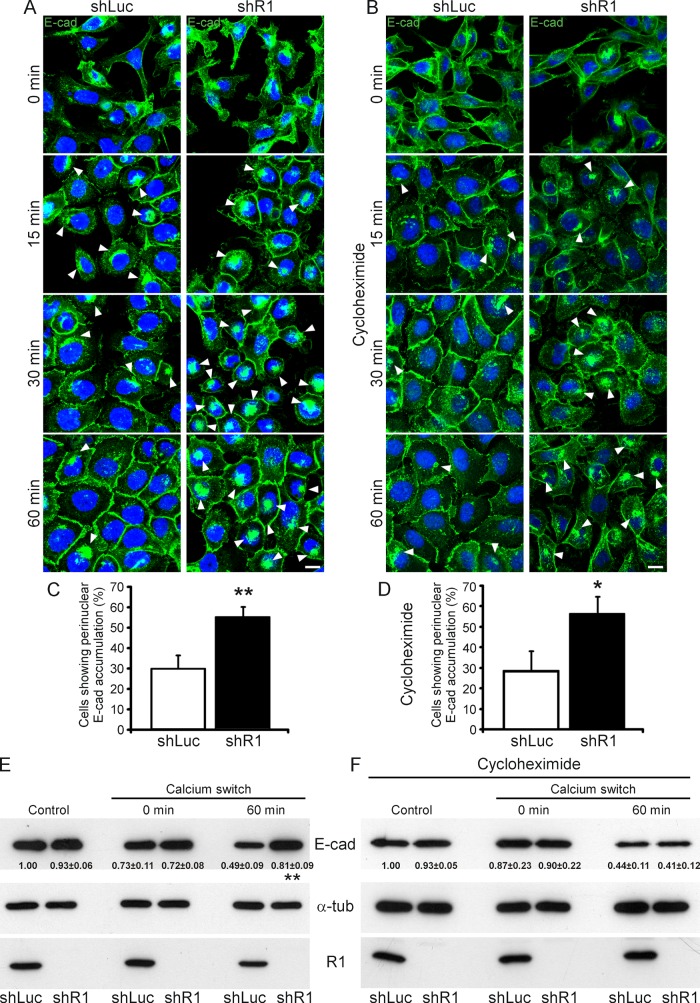
FIGURE 6:
Down-regulation of reggie-1 impairs E-cadherin recycling in A431 cells. (A, B) E-cadherin endocytosis and recycling were induced in shRNA stably transfected A431 cells by incubation with EGTA for 3 h, followed by a Ca2+ recovery for 0, 15, 30, or 60 min in the absence (A) or presence of cycloheximide (B). Depletion of Ca2+ and E-cadherin internalization causes cells to give up cell–cell contacts (0 min). Ca2+ repletion (15, 30, and 60 min) allows cell–cell contact reformation. Immunostaining using an E-cadherin (E-cad) antibody showed its strong perinuclear accumulation (arrowheads) at 0- and 15-min recovery in both cycloheximide-treated and nontreated reggie-depleted (shR1) and control (shLuc) A431 cells. Perinuclear E-cad accumulation, however, was strongly reduced after 30- and 60-min recovery in shLuc cells but maintained in the majority of shR1 cells independent of cycloheximide treatment (arrowheads). Incomplete formation of E-cadherin–mediated cell contacts during Ca2+ recovery in cycloheximide-treated shR1 cells was evident (B). Scale bars, 10 μm. (C, D) Quantification of the effect of reggie down-regulation on E-cadherin recycling in normal (C) and cycloheximide-treated (D) A431 cells (n = 3, *p < 0.05, **p < 0.01, paired t test; error bars, SEM). (E, F) Expression levels of E-cadherin, reggie-1 (R1), and α-tubulin (α-tub) as loading control were analyzed by Western blots from extracts of shLuc and shR1 A431 cells in absence (E) or presence of cycloheximide (F). shLuc and shR1 A431 cells (control) showed similar E-cadherin expression levels, which were slightly reduced after Ca2+ chelation (0 min) independently of cycloheximide treatment. After 60-min recovery, however, shR1 cells presented a significantly higher E-cadherin expression level than control shLuc cells (E). This effect was abolished upon cycloheximide treatment (F; n = 4, **p < 0.01, paired t test, mean ± SEM).