Scheme 7.
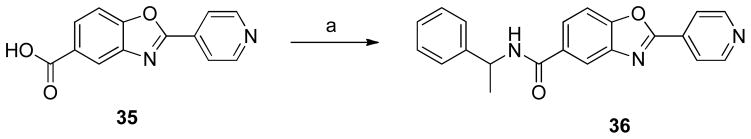
Synthesis of amide derivative 36.
Reagents and conditions: (a) α-methyl benzyl amine, EDCI•HCl, DMF, 0 °C - rt, 12 h, 71%. An analogue of 22 in which the amide group is inverted was prepared using the method outlined in Scheme 7. Amide 36 was generated by coupling 2-(pyridin-4-yl)benzo[d]oxazole-5-carboxylic acid (35) and α-methylbenzylamine in the presence of EDCI•HCl.
