Table 3.
SAR of the pyridine ring of 1 for CpIMPDH inhibition.
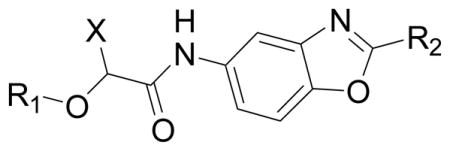
| |||||
|---|---|---|---|---|---|
| ID | X | R1 | R2 | IC50 (nM)
|
|
| (−) BSA | (+) BSA | ||||
| 59 | Me | 2,4-di-ClPh | Ph | >5000* | n.d |
| 60 | Me | 2,4-di-ClPh | 3-Py | 150 ± 20 | 600 ± 200 |
| 61 | Me | 2,4-di-ClPh | 2-Py | 210 ± 20 | 800 ± 200 |
| 62 | (S)-Me | 2,3-di-ClPh |
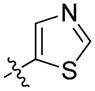
|
1.4 ± 0.3 | 2.3 ± 0.4 |
| 63 | (S)-Me | 1-naphthyl |
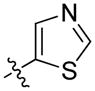
|
2.7 ± 0.7 | 4.0 ± 0.9 |
| 64 | Me | 1-(4-Cl-naphthyl) |
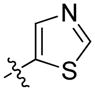
|
28 ± 6 | 70 ± 10 |
| 65 | (S)-Me | 2,3-di-ClPh |
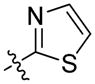
|
17 ± 6 | 30 ± 10 |
| 66 | (S)-Me | 1-naphthyl |
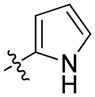
|
26 ± 5 | 50 ± 5 |
| 67 | Me | 1-naphthyl |
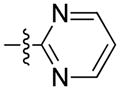
|
>5000* | n.d |
One determination, n.d. = not determined.
