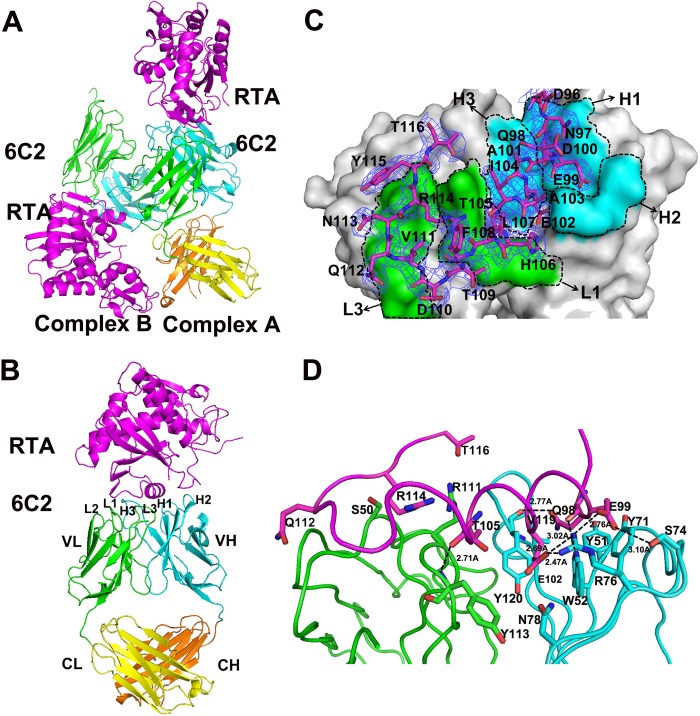
FIGURE 1.
Overall structure of the 6C2 Fab-RTA complex. A, crystal structure of 6C2 Fab-RTA complex in an asymmetric unit. B, crystal structure of 6C2 Fab-RTA (complex A). The RTA is colored magenta, the light chain (VL) and the heavy chain (VH) of 6C2 Fab are colored green and cyan, the light chain (CL) and the heavy chain (CH) of 6C2 Fab are colored yellow and orange, respectively, and the CDRs of 6C2 Fab are also labeled. C, surface of the 6C2 Fab-RTA complex and the relative role of each CDR loop in the interaction with the RTA. The residues on RTA are shown as sticks and colored magenta, the light chain (VL) and the heavy chain (VH) of 6C2 Fab are labeled and colored green and cyan, respectively. The 2Fo − Fc electron density map (contoured at 1σ) for the bound epitope peptide is shown as blue. D, binding interface between the 6C2 Fab and the epitope of the RTA. The important residues on the interface are shown as sticks, and hydrogen bonds are indicated by dashed lines. RTA and the CDRs are colored similarly on Fig. 1B.