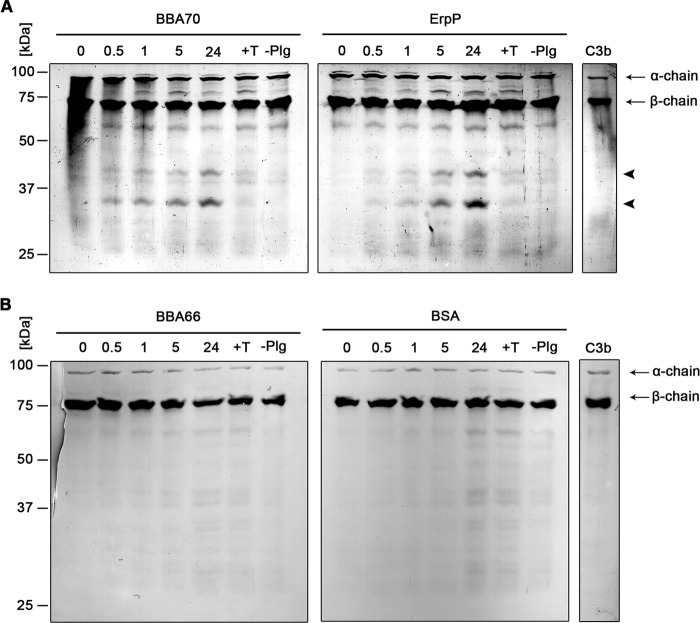
FIGURE 6.
BBA70-bound plasmin degrades complement component C3b. Microtiter plates were coated with recombinant, hexahistidine-tagged BBA70, ErpP, BBA66, and BSA (10 μg/ml). Following incubation with 10 μg/ml plasminogen, uPA (2.5 μg/ml) and purified C3b (5 μg/ml) were added. Microtiter plates were incubated at 37 °C for 24 h, and aliquots were taken at different time intervals. Following separation by SDS-PAGE, proteins were blotted onto nitrocellulose membranes, and C3b and its degradation products were detected, employing a polyclonal goat antiserum raised against C3b and a corresponding HRP-conjugated secondary antibody. Representative results of two independent experiments are shown. A, degradation of C3b by plasmin bound to BBA70 (left) and ErpP (right) after 0.5, 1, 5, and 24 h. Arrows, C3b α- and β-chain; arrowheads, C3b degradation products with a molecular mass of ∼30 and 40 kDa. B, results for BBA66 (left) and BSA (right). C3b, purified human C3b. Negative controls include the addition of 50 mm tranexamic acid (+T) as well as omission of plasminogen (−Plg).