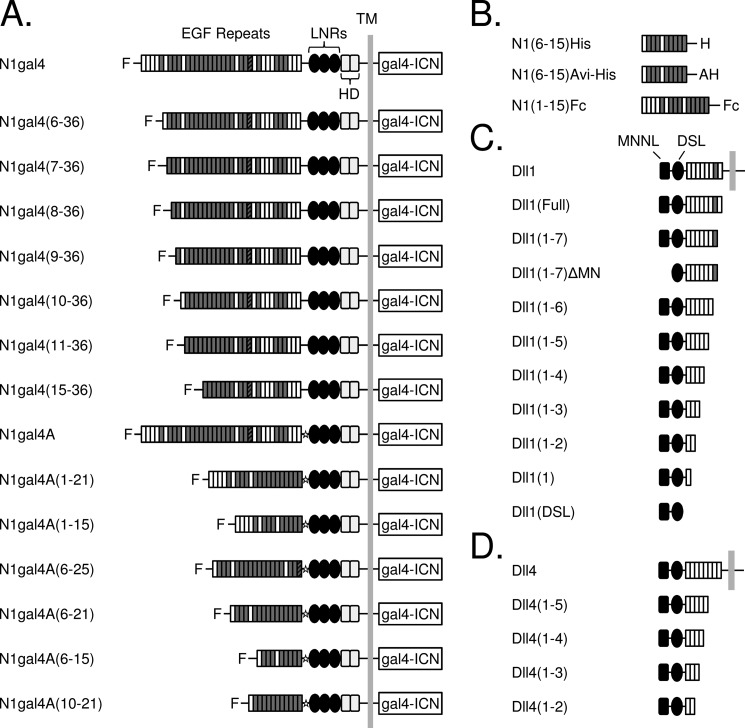
FIGURE 1.
Domain organization of ligand and receptor constructs. A, Notch molecules used in signaling assays. The N1gal4 construct was created by fusing a gal4 promoter to the intracellular region of Notch1 (gal4-ICN), and EGF repeats were systematically truncated from the N terminus. Repeats containing a consensus calcium-binding motif are shaded in gray, and EGF25, which contains an extra cysteine residue, is indicated with a striped pattern. “F” indicates the presence of an N-terminal FLAG epitope. C-terminal truncations were constructed using a Notch1-gal4A variant, which contains a restriction site (indicated with a star) between the EGF repeat region and the NRR. B, Notch molecules used in binding assays. The Notch extracellular truncation spanning EGF repeats 6–15, inclusive, was designed for use in protein-protein binding experiments, along with a N1(1–15)Fc fusion protein (R&D Biosystems). C and D, ligand molecules used in binding and signaling assays. C, human Delta-like 1 was systematically truncated from the C terminus, along with a variant lacking the N-terminal MNNL domain. D, C-terminal truncations of human Delta-like 4. LNRs, LIN12-Notch repeats; HD, heterodimerization domain.