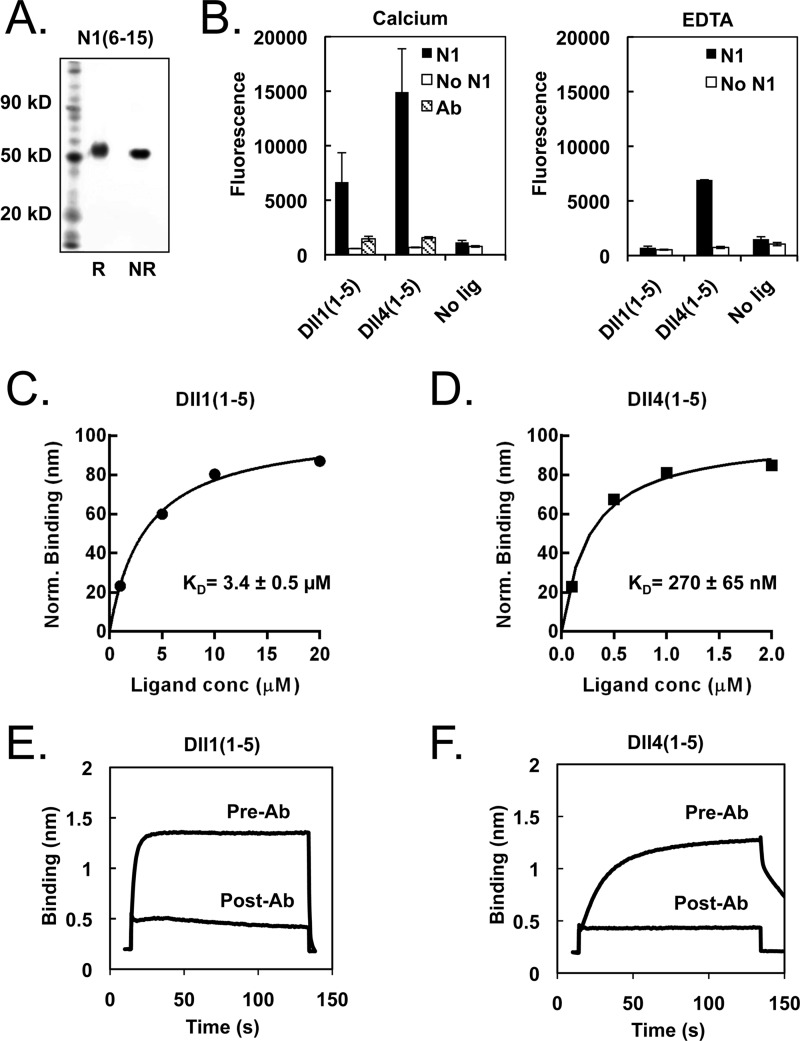
FIGURE 6.
Measurement of Notch-ligand interactions using purified proteins. A, SDS-PAGE gel of purified N1(6–15) protein under reducing and non-reducing conditions. B, ELISA binding assay. Functional ELISAs were performed on plated N1(6–15), bound with biotinylated Dll1(1–5) or Dll4(1–5) preclustered with Neutravidin-HRP. The resulting interaction is calcium-dependent and inhibited by blocking antibody. C and D, quantification of Notch-ligand binding affinity via biolayer interferometry (BLI). Biotinylated N1(6–15) was immobilized on a streptavidin biosensor and bound to varying concentrations of Dll1(1–5) or Dll4(1–5). The resulting concentration-response plots were fitted with a one-site exponential binding model and normalized for comparison, yielding KD values of 270 ± 65 nm for N1-Dll4 and 3.4 ± 0.5 μm for N1-Dll1 interactions. E and F, specificity of observed Notch-ligand binding. Treatment with the ligand-blocking antibody WC-613 (1 μg/ml) inhibited binding of Dll1 (5 μm) or Dll4 (2 μm) to N1(6–15).