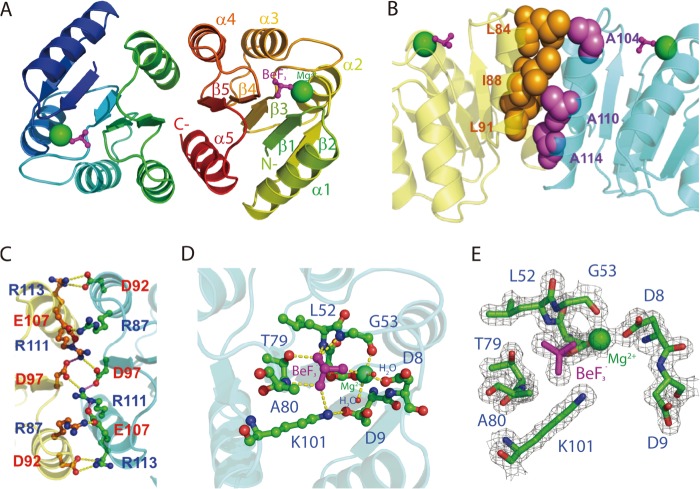
FIGURE 2.
X-ray crystal structure of PmrAN. A, ribbon structure of PmrAN shows the formation of a dimer with the interface at α4-β5-α5. The phosphate analog, BeF3−, is shown as a ball and stick figure in magenta, and Mg2+ is represented by a green sphere. B, hydrophobic interactions identified at the dimer interface. A hydrophobic patch (spheres) brings helices α4 (Leu-84, Leu-91, and Ile-88 colored in orange) and α5 (Ala-104, Ala-110, and Ala-114 colored in purple) together. C, the dimer interface is stabilized by an extensive network of salt bridges, Arg-87(α4)–Glu-107(α5), Asp-92(α4)–Arg-113(α5), and Asp-97(β5)–Arg-111(α5). D, an extended view of the active site. The Mg2+ is octahedrally coordinated to Asp-51, Gly-53, Asp-8, BeF3−, and two water molecules. The BeF3− moiety contacts with the side chain oxygen atom of Thr-79, the Nζ atom of Lys-101 and the backbone nitrogen atoms of Gly-53, Leu-52, and Ala-80. Carbon, oxygen, and nitrogen atoms are shown in green, red, and blue, respectively. E, active site electron density map (Fo − Fc) of the BeF3−-activated PmrAN calculated at 2σ is shown in gray within 1.7 Å of the residues depicted in sticks.