FIGURE 6.
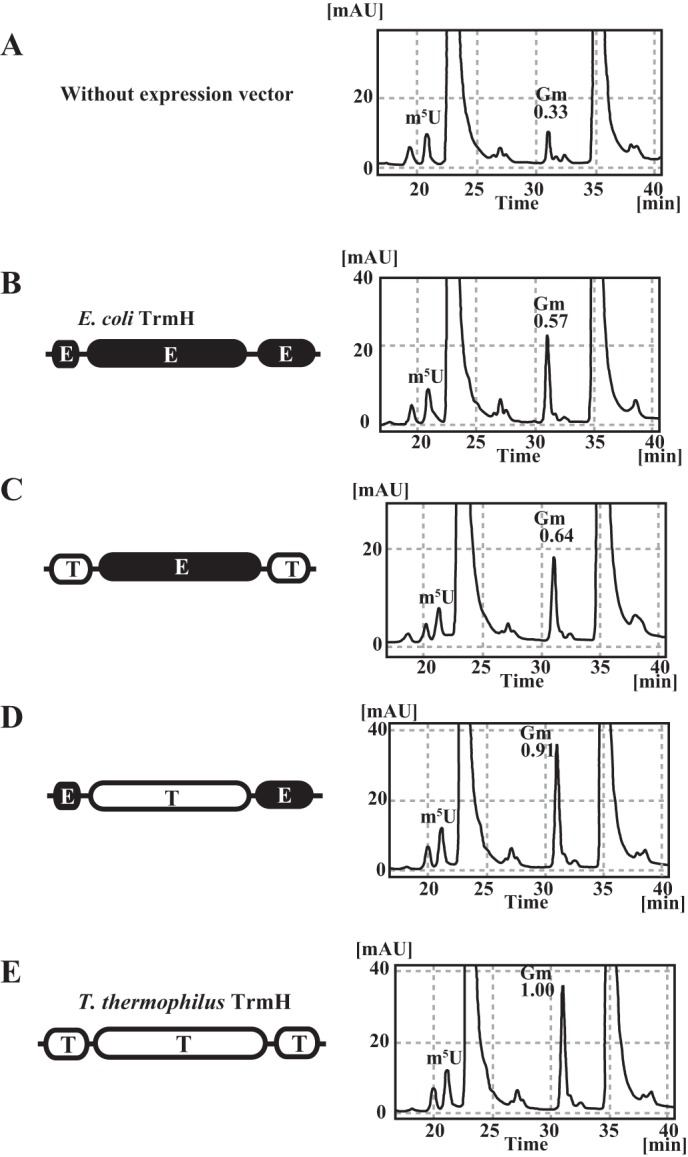
In vivo tRNA methylation activities of chimeric proteins. In the presence of endogenous E. coli TrmH (without expression vector), the Gm/m5U ratio in E. coli tRNA is 0.33 (A). When the wild-type E. coli TrmH was overexpressed, the ratio is raised to 0.57 but does not reach 1.00 (B). This result shows that limited tRNA species are methylated by E. coli TrmH and that the Gm18 modification in E. coli tRNA in natural conditions is a partial modification. When the chimeric protein, in which the catalytic domain is derived from E. coli TrmH, was expressed, the Gm/m5U ratio was close to the value from E. coli TrmH expression (C). Thus, this chimera protein methylates the limited tRNA species. In contrast, when the chimeric protein, which has the catalytic domain of T. thermophilus TrmH, was expressed, the Gm/m5U ratio was increased to 0.91, showing that this chimeric protein has broad substrate tRNA selectivity as compared with E. coli TrmH (D). When the wild-type T. thermophilus TrmH was expressed, tRNA was nearly fully modified (E). These in vivo experimental results suggest that the catalytic domain of E. coli TrmH distinguishes the substrate and nonsubstrate tRNAs, consistent with the in vitro results shown in Fig. 5.
