Figure 1.
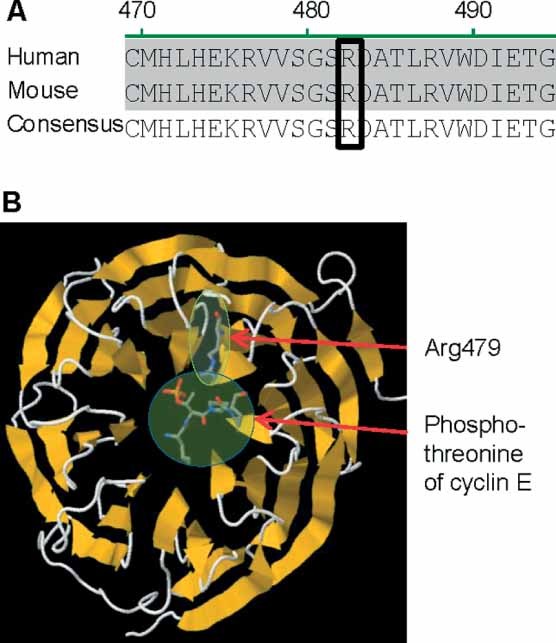
Fbxw7 alignment and molecular structure. (A) Alignment of FBXW7 protein sequence of human and mouse. The boxed region shows an arginine residue (human Arg479, mouse Arg482) in the WD40 domain which is commonly mutated in cancer patients. This residue is conserved between mouse and human FBXW7 and thus allows the generation of an informative mouse model. (B) Molecular model of FBXW7 WD40 propeller domain showing the effect of the human Arg479 mutation on the substrate binding site. The targeted arginine (human Arg479) is at the apex of one of the beta-sheets and interacts with the phosphothreonine of CCNE1 (an FBXW7 substrate). This interaction is predicted to be abolished if the arginine is mutated to glutamine. This protein model was generated using RasMol software
