Figure 3.
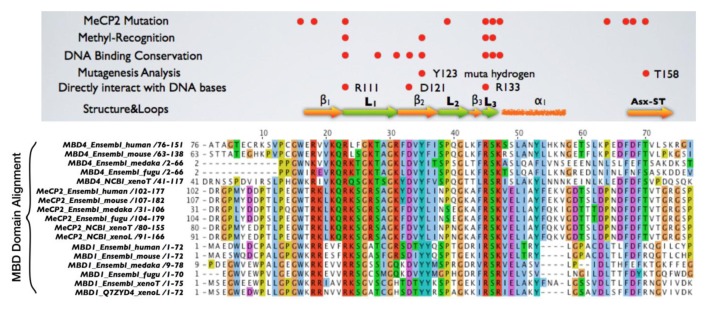
Key functional and structural amino acids are well conserved within MBD domain of MBD4. The MBD domain alignment is shown. A solution structure of the MBDs from MeCP2 and MBD1 has been determined, consisting of four anti-parallel β-strands, two of which were proposed to interact with the major groove of DNA, where a methyl group would be located. In addition, a number of conserved residues throughout the MBD domains of MeCP2 and MBD1 can be easily revealed by alignment, despite their full-length sequences sharing only moderate homology. The MBDs of MBD4, MBD1 and MeCP2 were aligned and compared to indicate essential residues within the MBD of MBD4 that are responsible for binding to methylated DNA sequences. Essential Residues are well conserved in the MBD of MBD4 [93]. In addition, amino acids within the MBD of MBD4 that are important for DNA binding found by mutational analyses and associated with Rett syndrome in MeCP2 are also well conserved. Sequence alignments of the MBD domain of MBD proteins were generated with clustalX module of Jalview software.
