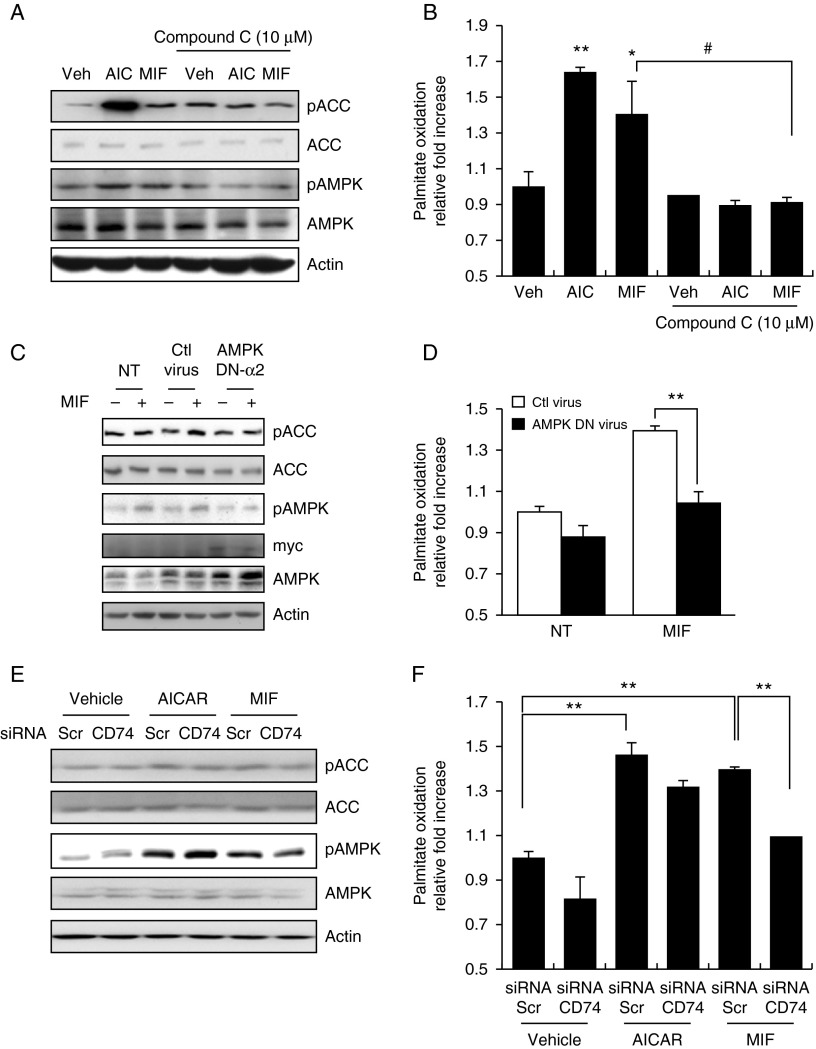
Figure 3.
MIF-stimulated palmitate oxidation in a CD74–AMPK-dependent manner. (A) HepG2 cells were pre-treated with compound C (10 μM) for 30 min and were then stimulated with MIF or AICAR for 1 h. Cell lysates were analyzed by western blotting with an anti-phospho-AMPK(Thr72) antibody. (B) Cells were pre-incubated with compound C and then stimulated under the indicated conditions. Oxidation of [3H]-labeled palmitate was measured as described in the Materials and methods section. (C) Cells infected with a mock adenovirus or an adenovirus carrying dominant-negative (DN)-AMPK α2 at an MOI of 30 for 18 h were treated with or without MIF. Cell lysates were analyzed by western blotting. DN-AMPK α2 expression was confirmed using an anti-Myc antibody. (D) Oxidation of [3H]-labeled palmitate was measured after infection with the mock or DN-AMPK a2 adenovirus. (E) HepG2 cells were transfected with CD74 or scrambled siRNA for 48 h and were then stimulated with MIF for 24 h. Whole-cell lysates were used to detect the phosphorylation of AMPK and ACC. (F) HepG2 cells were transfected with CD74 or scrambled siRNA for 48 h and were then stimulated with MIF for 24 h. HepG2 cells were incubated in 60 mm dishes for 24 h with either MIF or AICAR and were then assayed for oxidation of [3H]-labeled palmitate. Data are expressed as means±s.d. of triplicate experiments. *P< 0.05 vs vehicle; **P< 0.01 vs vehicle values and #P< 0.05 vs NT (compound C not treated) values (one-way ANOVA).