Abstract
Nitric oxide (NO) is an ubiquitous signaling molecule of intense interest in many physiological processes. Nitric oxide is a highly reactive free radical gas that is difficult to deliver with precise control over the level and timing that cells actually experience. We describe and characterize a device that allows tunable fluxes and patterns of NO to be generated across the surface upon which cells are cultured. The system is based on a quartz microscope slide that allows for controlled light levels to be applied to a previously described photosensitive NO-releasing polydimethylsiloxane (PDMS). Cells are cultured in separate wells that are either NO-releasing or a chemically similar PDMS that does not release NO. Both wells are then top coated with DowCorning RTV-3140 PDMS and a polydopamine/gelatin layer to allow cells to grow in the culture wells. When the waveguide is illuminated, the surface of the quartz slide propagates light such that the photosensitive polymer is evenly irradiated and generates NO across the surface of the cell culture well and no light penetrates into the volume of the wells where cells are growing. Mouse smooth muscle cells (MOVAS) were grown in the system in a proof of principle experiment, whereby 60% of the cells were present in the NO-releasing well compared to control wells after 17 h. The compelling advantage of illuminating the NO-releasing polymers with the waveguide system is that light can be used to tunably control NO release while avoiding exposing cells to optical radiation. This device provides means to quantitatively control the surface flux, timing and duration of NO cells experience and allows for systematic study of cellular response to NO generated at the cell/surface interface in a wide variety of studies.
Keywords: Tunable nitric oxide release, In vitro cell culture, Waveguide, Photolytic NO release, Timing and duration
Graphical abstract
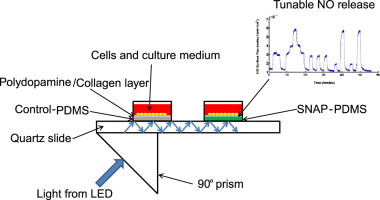
Highlights
-
•
Continuously controllable delivery of nitric oxide to cultured cells from the cell/surface interface.
-
•
Proves that light does not impinge upon cells in culture.
-
•
Provides the tool that enables systematic study of level, timing and duration of nitric oxide on cells.
Introduction
Over the past 25 years, nitric oxide (NO) has been shown to be a potent signaling molecule in a wide variety of physiological and pathological processes [1–7]. Nitric oxide has been shown to have anti-apoptotic effects in endothelial cells, lymphoma cells, ovarian follicles, cardiac myocytes and hepatocytes [2]. Alternatively, NO has been shown to possess pro-apoptotic properties in macrophages, neurons, pancreatic β-cells, thymocytes, chondrocytes and hepatocytes [3]. There are a number of reviews that suggest NO in low levels has a protective and proliferative effect on cells while at high levels induces cell cycle arrest, apoptosis and senescence [1,6,7]. The contradictory effects of NO are dependent in part on a number of factors including flux of NO, the timing of NO release, the accumulated dose of NO, and the type of cells exposed to the NO.
There are several factors that contribute to challenges in determining the level and duration of NO that actually causes these various affects using conventional soluble NO donors as the means by which NO is introduced to cells. First, because NO is a highly reactive molecule, it is difficult to know what level of NO, the cells, are actually experiencing when soluble NO donors are added to culture media. It is nearly impossible to relate a bulk concentration of added donor to the level of NO the cells actually experience. The depth and volume of the culture medium, the mixing rate, temperature and specific content of the culture medium profoundly influence the rate of donor decomposition and therefore rate of NO release. Additionally, there is little control over the temporal release of NO from systems that inject donors that decompose to release NO as soon as they encounter aqueous solutions such as media or dissolved ions that initiate NO release. A much better assessment of what level of NO the cells may experience is surface flux in units of mol per surfaces area per time (e.g., mol cm−2 min−1). For example, at the vessel wall/blood interface, NO is generated at the vessel surface by the endothelial cells lining, platelets close to the endothelial cells experience a higher flux of NO than platelets in the center of the vessel lumen. In order to assess what level of NO is needed to prevent platelet adhesion and activation, it is more important to know how much NO is present at the endothelial surface rather than the average concentration in the bulk of blood. It has been estimated that 0.5–4×10−10 mol cm−2 min−1 of NO is produced by endothelial cells that line healthy blood vessels [8]. This information is not dependent on the overall vessel geometry or volume of blood, whereas the concentration of NO measured in blood is the average amount of NO produced with no means to account for the concentration gradient that exists from the vessel wall towards the center of the lumen.
Studies from several groups have evaluated materials prepared with chemistries that release or generate NO from their surfaces at constant, continuous fluxes [9–12]. These studies found that in blood contacting applications, NO release led to significant reductions in overall gross thrombus formation compared to control materials, thereby demonstrating the efficacy of NO in mediating aspects of the host response to implanted probes [13,14]. However, a key limitation to this strategy exists in that once NO release is initiated there is no means to alter the NO surface flux. This limits our ability to understand how much and in what durations NO is needed to control biological response, such as thrombus formation. The continuous release also limits the total duration of effective NO generation capability (i.e., NO may be released when it is not needed, depleting the finite reservoir of NO available in the NO-release coating and shortening the potential life time of the implant). A method to variably control NO release and use an on/off trigger for NO generation could provide a tool by which NO release can be restricted to physiological needs, potentially extending the functional life of an implanted probe. We have recently reported the synthesis of polydimethylsiloxane (PDMS) that contains the light sensitive NO donor, S-nitroso-N-acetyl-d-penicillamine (SNAP), covalently linked to the crosslinking groups used to make the polymer (material referred to as SNAP–PDMS) [15]. This polymer was coated onto optical fibers and demonstrated highly tunable release by controlling the light used to illuminate the optical fiber [16]. The continuously variable, controlled release of this material offers a means to begin systematically studying the effects of both the surface flux of NO generated and the timing of NO release on cellular response.
Herein, we describe and characterize a novel planar waveguide system that utilizes SNAP–PDMS as the bottom of wells such that tunable fluxes and patterns of NO can be generated across the surface of the polymer upon which cells can be cultured. The actual solid core waveguide is a quartz microscope slide that is housed by a custom built, milled aluminum housing with 1.5 cm diameter Teflon® rings used to form culture wells on the surface of the slide. Light is directed along the core of the slide by 90° prisms and optical silicone coupling oil. The slide is illuminated along the entire surface and produces a leaky evanescent wave that is sufficient to generate NO from the photosensitive SNAP–PDMS cast in the bottom of the culture wells. A control well that does not release NO is also included on the waveguide by casting the modified PDMS that has not been nitrosated (NAP–PDMS) in a second Teflon® ring. Both the NO releasing SNAP–PDMS and the control NAP–PDMS were top coated with DowCorning RTV-3140 and a polydopamine/collagen layer to allow cells to grow in the culture wells. Thus, all cells are exposed to the same culture conditions and durations of light exposure with the only difference being the presence or absence of NO. A compelling advantage of illuminating the NO-releasing polymers in conjunction with the waveguide is that light can be used to tunably control NO release while avoiding exposing cells under study to optical radiation. This device provides a means to quantitatively control the surface flux, timing and duration of NO cells experience and allows for systematic study of cellular response to NO generated at the cell/surface interface.
Materials and methods
Solid core waveguide device
Uncoated high tolerance right angle prisms (25 mm) were obtained from Edmund Optics (Barrington, NJ, USA). Quartz slides (75×25×1 mm3) were obtained from Chemglass Life Sciences (Vineland, NJ, USA). The VAOL-5GSBY4 LED (λdominant=470 nm) and VAOL-5GWY4 LED (white light) were purchased from Mouser Electronics, Inc (Mansfield, TX, USA) and the SSL-LXTO46UV1C UV LED (λdominant=385 nm) and SSL-LXTO46UV2C (λdominant=405 nm) were obtained (VCC Optoelectronics, San Marcos, CA); all fixed within Amphenol SMA receptacles (Wallingford, CT, USA). A USB 4000 fluorometer, USB 4000 UV–vis spectrometer, P-6000-2-UV/vis optical fiber, CUV-ALL-UV 4-way Cuvette Holder and c74-UV collimating lens (200–2000 nm) were all purchased from Ocean Optics (Dunedin, FL, USA). Raw data recorded from the fluorometer was in counts striking the detector, therefore all results were normalized to 200 ms collection time and the highest value detected in order to allow comparison of data. Fluoroscein isothiocyanate dextran and dextran were purchased from Sigma (St. Louis, MO, USA). The phosphate buffered solution (PBS) was made in-house at 9.4 pH. Silicon rubber sheet (1 cm thick) was purchased from McMaster Carr (Elmhurst, IL, USA) and cut into 1.3 cm by 5.8 cm wells and adhered to a quartz slide by the manufacturer adhesive backing. Data collected from the spectrometer was calibrated first in the dark and then in full light from a white LED with a cuvette in the cuvette holder and light passing straight though the cuvette allowing for absorbance to be measured. Gafchromatic EBT3 Dosimetry Film was purchased from Ashland, Inc. (Wayne, NJ, USA). The RX Firefly UV Light System (λdominant=400 nm) from Phoseon Technology (Hillsboro, OR, USA) and plastic cuvettes were purchased from VWR (Arlington Heights, IL, USA).
SNAP–PDMS synthesis
Hydroxy-terminated 2000 cSt polydimethylsiloxane (PDMS) was purchased from Gelest, Inc. (Morrisville, PA, USA). 3-Aminopropyl trimethyoxysilane, dibutyltin dilaurate and toluene were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). Tert-butylnitrite (90% technical grade) (t-BuONO) was purchased from Acros Organics (Geel, Belgium). All reagents were used as received except the t-BuONO. The t-BuONO was first cleaned by extracting over 30 mM aqueous cyclam (Sigma-Aldrich) in order to remove copper added as a stabilizing agent.
Detailed synthesis of this materials has been described elsewhere [15], briefly 1.6 g of hydroxy-terminated PDMS was mixed with 0.3 g of 3-aminopropyl trimethoxysilane, 2.4 mg of dibutyltin dilaurate and 8 mL of toluene. The solution was stirred for 24 h to crosslink the PDMS. A self-protected thiolactone was synthesized according to the method by Moynihan [17]. Fifty milligram of this thiolactone was then mixed with 1 mL toluene followed by 2 mL of the crosslinked PDMS. The solution was stirred for an additional 24 h to allow the thiol-containing compound to react with the primary amine groups present on the cross-linking agent. One milliliter of cleaned t-BuONO was then added and the polymer and was protected from light and placed on a shaker table for 15 min which resulted in an emerald green polymer. This color change is indicative of the formation of SNAP linked to the PDMS backbone (final product is SNAP–PDMS). After shaking, 500 μl of the polymer solution was cast in a 1.5 cm i.d. Teflon® ring secured to a quartz slide and allowed to cure in the dark under ambient conditions. Likewise, a control well was cast with the t-BuONO being replaced with toluene. Both samples were then coated with 500 μl of a solution containing DowCorning RTV-3140 in toluene (1 g/10 mL) (Ellsworth Adhesives, Germantown, WI, USA).
Measuring NO surface flux
The SNAP–PDMS film, which was cast onto the quartz slide inside 1.5 cm diameter Teflon® rings, was placed within the custom-milled aluminum holder and connected to a Seivers Nitric Oxide Analyzer (NOA) 280i (GE Instruments, Boulder, CO, USA). The entire sample holder was protected from ambient light. Nitrogen gas was used as the sweep gas. Raw data was collected in PPB and converted to surface flux by multiplication with a calibration factor experimentally determined for the instrument by the acidification of sodium nitrite (calibration factor for these experiments was 8.76×10–14 mol s−1 PPB−1) divided by surface area of the material.
Polydopamine-coating
SNAP–PDMS and NAP–PDMS coated wells that were top coated with RTV-3140 were coated with polydopamine and gelatin layer before cell seeding. Dopamine hydrochloride (Sigma-Aldrich, St. Louis, MO) was dissolved in 10 mM Tris-buffer (pH=8.5) (Sigma-Aldrich St. Louis, MO) to the final concentration of 2 mg/ml. PDMS films were submerged in dopamine solution open to atmosphere. Coating process lasted for 2 days. The coated PDMS wells were cleaned by 2 min of sonication in deionized water and rinsing with deionized water. The coated PDMS was treated by 2 mg/ml collagen gelatin (EIA Grade Reagent Gelatin, Bio-Rad, Hercules, CA) solution for 1 h and then used for cell seeding.
Smooth muscle cell culture
The mouse aorta smooth muscle cell line (MOVAS-1) was purchased from American Type Culture Collection (ATCC), (Menassas, VA, USA). MOVAS were cultured in ATCC formulated Dulbecco's Modified Eagle Medium (DMEM) with 0.2 mg/ml G418 from ATCC (Menassas, VA, USA), 1% penicillin–streptomycin from ATCC (VA, USA) and 10% fetal bovine serum (FBS) from ATCC (Menassas, VA, USA). The cell number was measured by a hemocytometer. All the cells were seeded at a density of 2.5×102/mm2. Cells were incubated in humidified 5% CO2, 37 °C incubator. Cells were stained by calcein-AM (Sigma-Aldrich, St. Louis, MO, USA) and imaged with an Olympus BX51 fluorescent microscope.
Statistical analysis
Data was analyzed by Student's t-test and/or one-way analysis of variance (ANOVA). The P-value of 0.05 or less is considered as a statistically significant difference.
Results and discussion
Characterization of the solid core waveguide device
The propagation of light through the waveguide device was confirmed and the potential of light penetrating into the culture well was investigated. In order to determine if light was penetrating into the volume of the culturing well, two tests were completed. First, the 1.3×5.7 cm2 silicon rubber well was placed on the quartz waveguide and filled with fluorescently labeled dextran solution to determine if any fluorescence was detected when the excitation wavelength was used to illuminate the waveguide. Second, the light sensitive film Gafchromic EBT3 was used to detect the accumulated light energy that penetrated the well volume over the length of the in vitro culture test and to assess the evenness of illumination across the wells.
Fig. 1 illustrates the prism arrangement and silicone rubber well that was created to assess light propagation and depth of penetration into the volume of the sample well created on the waveguide. Fig. 2 panel 1 shows the propagation of light from a 470 nm LED through an uncoated quartz slide (i.e., no SNAP–PDMS, RTV-3140 or collagen were applied) with USB 4000 fluorometer detecting light exiting the waveguide. With no slide is in position, light is not directed into the prism that couples light to the spectrometer (trace A), when the slide is in place with silicone optical coupling gel, light is propagated through the waveguide (trace B). A PBS or PBS with dextran solution was placed on the surface of the waveguide and light propagated through the device decreases to 0.25 of the original value (traces C and D, respectively, which are overlapped) because light interacts with the material directly in contact with the waveguide. When the PBS solution contained fluorescently labeled dextran (λex=470 nm, λem=540 nm), a fluorescence emission at 540 nm was detected by the fluorometer coupled to the exiting prism that had a relative intensity of 0.06 of the original light detected from the waveguide (trace E). The observation that the amount of light propagated decreased when PBS was in contact with the surface and that fluorescence emission was detected when labeled dextran was in the sample well both confirm that the material closest to the waveguide surface interacts with the light used to illuminate the waveguide.
Fig. 1.
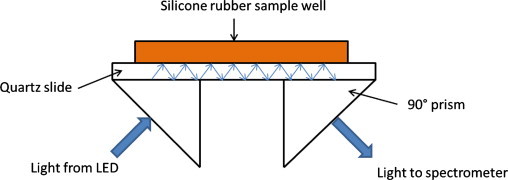
Schematic diagram of the prism configuration used to illuminate the quartz slide which serves as the solid core waveguide (optical coupling gel was used between prisms and slide). Light from an LED is directed into the quartz microscope slide that serves as a waveguide and is detected by a spectrometer coupled to the exit prism. A sample well was created by cutting out a 1×1.3×5.7 cm3 block from silicone rubber and adhering it to the quartz slide.
Fig. 2.
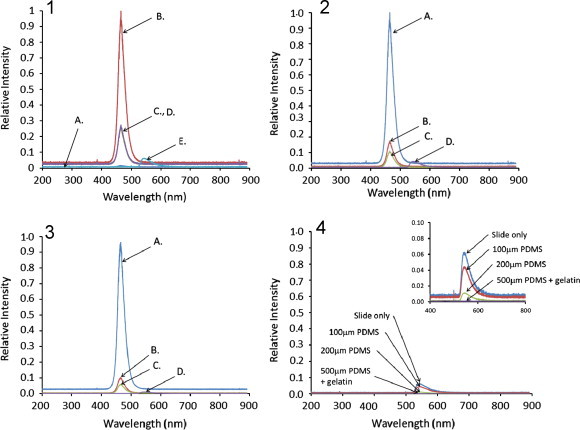
Relative level of light propagated through the quartz slide waveguide when different layers of PDMS were used to coat the waveguide. Panel 1 shows light propagation through a bare quartz slide where trace (A) no slide is connecting the prisms, (B) slide with no liquid on the surface, (C) slide with PBS on surface, (D) slide with PBS+dextran is on the surface and (E) slide with PBS and fluoroscein isothiocyanate dextran is on the surface. Panel 2 shows light propagated through a quartz slide with 100 μm thick layer of DowCorning RTV-3140 when (A) slide with no liquid on the surface, (B) slide with PBS on surface, (C) slide with PBS+dextran is on the surface and (D) slide with PBS and fluoroscein isothiocyanate dextran is on the surface. Panel 3 shows light propagated through the quartz slide waveguide with 200 μm thick layer of DowCorning RTV-3140 when (A) slide with no liquid on the surface, (B) slide with PBS on surface, (C) slide with PBS+dextran is on the surface and (D) slide with PBS and fluoroscein isothiocyanate dextran is on the surface. Panel 4 shows a comparison of the fluorescent signal generated from the fluoroscein isothiocyanate dextran with a bare quartz slide only, a ~100 μm layer of DowCorning RTV-3140 (PDMS), a ~200 μm layer of PDMS and ~500 μm layer of PDMS with polydopamine/gelatin. As the layer thickness increased, the interaction of light from the waveguide with the bathing solution decreased and no interaction was observed with the PDMS with polydopamine/gelatin. Inset shows region of interest expanded. A 470 nm LED was used to illuminate waveguide in all cases.
To determine how far away from the surface of the waveguide light will propagate (i.e., how far into the volume of cell culture well light will penetrate), sets of films were created by casting 1 or 2 layers of 500 μL/cm2 well surface area of the RTV-3140 PDMS solution the silicone rubber well on the waveguide (single and double cured layers were ~100 μm and ~200 μm thick were achieved). Additionally, films created by casting 2 layers of PDMS with a top coating of polydopamine/gelatin (total thickness of all layers ~500 μm) to replicate of the functional culture system was cast in the well on the waveguide surface. Fig. 2 panel 2 shows light propagated through the waveguide with 100 μm thick RTV-3140 coating (trace A), the addition of PBS on top of the film has a normalized intensity of 0.16 (trace B), PBS with 0.167 mM dextran has a relative intensity of 0.09 (trace C), and the relative intensity of light propagated through the waveguide with 0.167 mM fluorescent dextran in the PBS is 0.04 (trace D). Fig. 2 panel 3 shows light propagated through the waveguide with a 200 μm thick RTV-3140 coating (trace A), PBS placed on top of the film has a normalized intensity of 0.09 (trace B), PBS with dextran has a relative intensity of 0.05 (trace C), and the relative intensity of light propagated through the waveguide with 0.167 mM fluorescent dextran in the PBS is 0.01 (trace D). All values were normalized by dividing the intensity counts recorded by the fluorometer by the intensity counts of each respective film.
Fig. 2 panel 4 compares the fluorescent emission detected from the fluorescently labeled dextran solution on bare quartz slide (relative intensity ~0.06), on top of a ~100 μm thick PDMS layer (relative intensity ~0.04), on top of a ~200 μm thick PDMS layer (relative intensity ~0.01) and on top of the ~500 μm thick PDMS layer with polydopamine/gelatin (no emission detected). Because the fluorescence intensity decreases with increasing separation from the surface of the waveguide and no fluorescence was detectable with the addition of the gelatin layer, the light that is propagated in the waveguide is primarily interacting only with the layer closest to the surface. There is no interaction of light with the sample that is raised ~500 μm off the surface of the waveguide with polydopamine/gelatin layer. This demonstrates that the cells within the volume of the wells on the waveguide will not experience irradiation by the light source used to initiate and control NO release from the SNAP–PDMS.
The evenness of the illumination of the NO-releasing polymer in the bottom of the cell culture wells directly determines how uniform the delivery of NO will be to the cells grown in the culture well. To assess evenness of illumination a 18 mm square piece of Gafchromic EBT2 photosensitive film was centered over each 90° prism and placed directly on a bare quartz slide with optical coupling oil. The waveguide was illuminated with a 405 nm dominant LED (dive current 50 mA) coupled to a UV/Vis optical fiber. This system was then exposed for 30 h. Likewise, an 18 mm sample was placed over the entrance of the LED light, prior to light entering the prism, and exposed for 30 h. A similar piece of film was then exposed to 405 nm, 8 W light from a RX Firefly UV light for 5 min at 1 cm from the light source. This was used to show that the film was changing with respect to light at the desired wavelengths. The films were then cut into 9 sections and adhered to a disposable cuvette using optical coupling oil such that the entirety of the white LED light was on the sample (approximately 1.5 cm from the bottom of the cuvette). It should be noted that the white LED was used as the source for the UV–visible absorbance spectra because it contains very little 405 nm light and will therefore not cause continued absorbance changes in the Gafchromic EBT3 film while recording spectra of the exposed films. The UV–vis absorbance spectrum was then collected for each sample as well as an unexposed, control piece. The peak of interest occurred at 636 nm and was compared to a non-changing peak between samples at 470 nm for each section of the film. Fig. 3A shows the relative change in absorbance after 5 min of bulk irradiation from the RX Firefly light source 1 cm from the surface of the film. Fig. 3B shows the relative change in absorbance of the film exposed to the light entering the prism. This shows that the light entering the prism is strong enough to cause a noticeable change in absorbance and that the light is stronger in the middle of the prism than on the edges (i.e., the center of the film showed more change in absorbance than edges of the film). Fig. 3C and D shows the relative absorption changes for the films in the culture setup, coupled to the quartz slide on the waveguide system near the entry point of the light sources (1.2 cm from the end of the slide) and further from the light source (4.7 cm from the end of the slide) illuminated for 30 h. The films show uniform change in absorbance across the surface of the film. This evidence indicates two import aspects of the waveguide system. First, the surface of the waveguide system is indeed evenly illuminating the surface of the quartz slide, which will evenly release NO from the SNAP–PDMS used as the base of the cell culture wells. Secondly, it indicates that very little light is interacting with the layer closet to the surface of the waveguide. The photosensitive films extensively changed color after 5 min of direct exposure from the RX Firefly UV light source, however after 30 h of illumination on the waveguide system, the films changed very little. This data in conjunction with the fluorescence data clearly indicate that no optical radiation is impinging on the cells grown within the culturing wells.
Fig. 3.

Maps that show the relative change in absorbance across the surface of Gafchromic film illuminated with (A) 405 nm bulk irradiation from the RX Firefly light source 1 cm from the surface of the film for 5 min, (B) 405 nm light from LED immediately exiting the prism for 30 h, (C) 405 nm LED light from the waveguide system 1.2 cm from the end of the slide for 30 h, and (D) 405 nm LED light from the waveguide system 4.7 cm from the end of the slide illuminated for 30 h.
Design of the solid core waveguide device
Fig. 4 shows the schematic of the solid core waveguide device that allows NO to be generated in a controlled manner across the surface upon which cells are cultured. The solid core waveguide is a quartz microscope slide. In order to grow cells on this device, wells were formed by affixing Teflon rings to the face of the quartz slide and casting SNAP–PDMS or NAP–PDMS in the bottom of the wells. Light was directed along the core of the slide by 90° prisms coupled to the slide by optical silicone coupling oil. Light propagated through the slide and illuminated the entire surface of the slide with a leaky evanescent wave that was sufficient to generate NO from the photosensitive SNAP–PDMS. The light source used was either a 470 nm LED (to characterize light penetration into the volume of the cell culture well) or UV-LED (to generate NO during the cell culture experiment). As the drive current applied to the LED was increased, the power of the light produced also increased, in turn releasing more NO. This provides a means by which controlled NO delivery to the cells growing in the culture well is achieved.
Fig. 4.
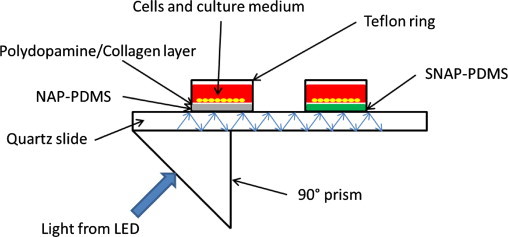
Schematic diagram of the solid core waveguide device that has the ability to deliver precise levels of NO to cells cultured upon it in the wells of the device. Light from an LED is directed into the quartz microscope slide that serves as a waveguide. SNAP–PDMS cast in the bottom of the wells photolytically releases NO in response to the level of light administered by the LED, thus exposing cells to controlled levels of NO.
The well side of the SNAP–PDMS was top coated with DowCorning RTV-3140 PDMS and a polydopaimne layer was deposited on the PDMS. Collagen gelatin was then cast on the polydopamine layer. This gelatin layer was the actual surface the cells contacted. The polydompamine layer effectively interfaced the hydrophobic PDMS and the hydrophilic gelatin, allowing a surface to be created upon which cells are normally cultured.
Fig. 5 demonstrates the potential for tune-ability in the NO surface flux generated at 22 °C in response to illumination of the waveguide with a SSL-LXTO46UV1C UV LED (λdominant=385 nm) with an applied potential of 6 V and varied resistance over a range of 90–200 Ω. This results in changes of the drive current (Idrive) applied to the LED in the range of 25–90 mA. Table 1 lists the sequential Idrive applied to the UV LED and the corresponding NO surface flux generated as measured by chemiluminescence. Small changes in Idrive (25 mA–30 mA) resulted in measureable change in the surface flux of NO, demonstrating fine control over the level of NO generated.
Fig. 5.
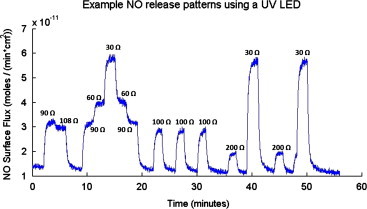
Demonstration of the tunability in the NO surface flux generated in response to illumination of the waveguide with a SSL-LXTO46UV1C UV LED (λdominant=385 nm) with an applied potential of 6 V and resistance varied in the range of 90–200 Ω. NO release was measured by chemiluminescence at 22 °C.
Table 1.
Listing of the sequential resistance used with a constant applied potential of 6 V and the resultant drive current (Idrive) used with the SSL-LXTO46UV1C UV LED (λdominant=385 nm) to generate nitric oxide (NO) from SNAP–PDMS on the waveguide for trace shown in Fig. 6. Applied potential was turned off when “off” listed in resistance column, resulting in no light illuminating the waveguide.
| Resistance used (Ω) | Resultant Idrive (mA) |
|---|---|
| Off | |
| 90 | 30 |
| 108 | 25 |
| Off | – |
| 90 | 30 |
| 60 | 45 |
| 30 | 90 |
| 60 | 45 |
| 90 | 30 |
| Off | – |
| 100 | 27 |
| Off | – |
| 100 | 27 |
| Off | – |
| 100 | 27 |
| Off | – |
| 200 | 13.5 |
| Off | – |
| 30 | 90 |
| Off | – |
| 200 | 13.5 |
| Off | – |
| 30 | 90 |
| Off | – |
Preliminary cell experiments with MOVAS
To demonstrate that cells will indeed grow in the culture wells of the waveguide device on gelatin layers interfaced with PDMS films by polydopamine with the use of UV light as a trigger for NO release. As a proof-of-concept experiment, mouse vascular smooth muscle cells (MOVAS) were cultured in the waveguide device (n=3) and on a glass slip cover coated with collagen gelatin in a standard culture plate (n=3). These cells were selected as the test to demonstrate feasibility because growth and proliferation of MOVAS are well known to be inhibited by NO [18,19]. Cells were cultured for 17 h at 37 °C in a humidified 5% CO2 incubator. The UV LED used to initiate NO release from the SNAP–PDMS was illuminated for the entire duration of the experiment. The real-time NO release from the SNAP–PDMS containing cell culture well (representative release shown in Fig. 6) was measured using chemiluminescence for a duplicate waveguide (i.e., same SNAP–PDMS/polydopamine/gelatin layer and sterilization methods but without cells and culture medium) incubated at cell culture conditions. Fig. 7 shows representative images of the results of the cells cultured under these conditions. The control grown under standard culturing conditions (gelatin coated glass coverslip) (Fig. 7A) and the control on the waveguide device (non-NO releasing PDMS layers with polydomamine/gelatin layers) (Fig. 7B) both show the same cellular proliferation, while the cells cultured on the NO releasing surface show reduced proliferation (Fig. 7C). Fig. 7D shows the bar graphs of the relative cell numbers in each set of culture conditions. Both sets of control conditions, non-NO releasing well on the waveguide with continuous illumination of the waveguide with UV light from UV LED and the control in standard conditions show no statistically significant difference in the relative number of cells. The cells grown on the NO releasing SNAP–PDMS surface showed less cellular growth and proliferation with approximately 60% the number of cells as the controls (p<0.05). These results also show that the NO released from the SNAP–PDMS culture well inhibited growth of the cells grown upon it and did not affect the cells grown in the neighboring control well on the waveguide device. It should be noted that the level of NO generated in this system was not tuned or altered during the course of the experiment (i.e., the applied drive current to the UV LED was not modulated). Because the cells were not effected by 17 h of continuous illumination with UV LED, this opens the possibilities of completing detailed studies concerning the effects of precisely controlled level, timing and duration of NO exposure to a wide variety of cells by modulating the light from the UV LED.
Fig. 6.
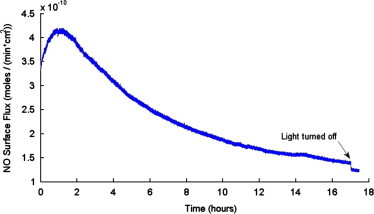
NO surface flux that MOVAS cells experienced when waveguide was illuminated with the SSL-LXTO46UV1C UV LED (λdominant=385 nm) with 70 Ω resistance and an applied potential of 6 V over a 17 h period measured with chemiluminescence at 37 °C, upon termination, the light was turned off and NO production abruptly dropped which shows that NO is still controlled.
Fig. 7.
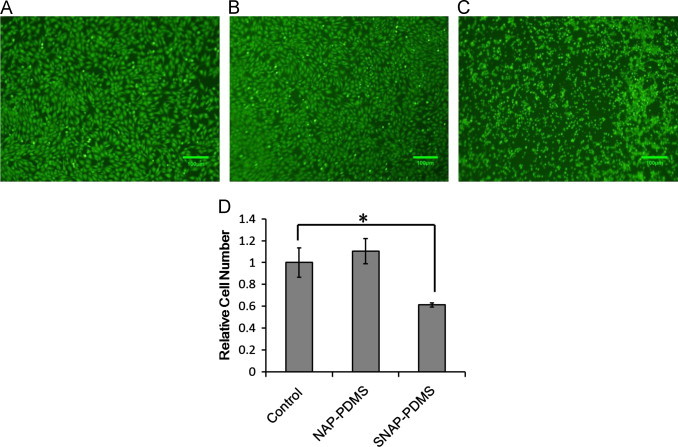
Representative images of MOVAS cells cultured for 17 h at 37 °C in a humidified 5% CO2 incubator on (A) gelatin layer cast on glass slip cover under standard culture conditions, (B) on a gelatin layer on top of non-NO releasing PDMS in waveguide device and (C) on gelatin layer on top of SNAP–PDMS in waveguide device. Waveguide was continuously illuminated UV LED (λdominant=385 nm, 70 Ω resistance and 6 V applied potential) which resulted in continuous NO release from the SNAP–PDMS for the entire duration of the experiment. Panel (D) is a histogram of the relative number of cells observed on the different surfaces. There is no statistically significant difference in the number of cells on the control and non-NO releasing PDMS surfaces and there is a statistically significant difference in the relative number of cells on the SNAP–PDMS surface (60%) the relative number of cells compared to the control surface (p<0.05).
Additionally, to prove that the inhibition of proliferation observed in the SNAP–PDMS well on the waveguide resulted only from NO released, we completed a 5 day culture of the MOVAS cells on SNAP–PDMS and NAP–PDMS top coated with RTV-3140, polydopamine and collagen gelatin as described for the experiment completed on the waveguide device, but cultured the cells in the sample wells in the dark (therefore preventing photoinitiated NO release). Fig. 8 shows the cells have grown to confluence on both the surface that has the potential to release NO and the control surfaces. This is confirmation that components from the underlying PDMS/polydopamine layers are not causing cytotoxicity affects to the cultured cells.
Fig. 8.
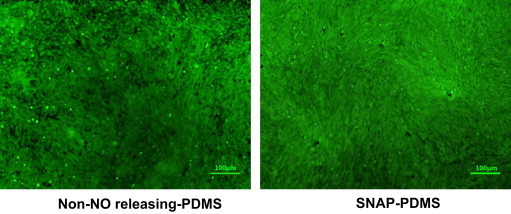
Representative images of MOVAS cells cultured for 5 days at 37 °C in a humidified 5% CO2 incubator on (A) a collagen layer on top of NAP–PDMS and (B) on collagen layer on top of SNAP–PDMS kept in the dark to prevent NO release. Both cultures grew to confluence, indicating that the PDMS/polydopamine layers under the collagen the cells are cultured upon are not causing cytotoxicity.
The purpose of these experiment was to demonstrate that cells can be grown on the waveguide device, the control cells are not affected by the constant UV light illuminating the core of the waveguide device and that NO is indeed generated in the well containing the photosensitive NO releasing polymer. We demonstrated that optical radiation from the waveguide does not penetrate the volume of the cell culture wells and that the surface of the waveguide is evenly illuminated. Additionally, the cells grown in the control culture well on the waveguide device were indistinguishable from the control culture that was carried out under standard culture conditions. This validates that the control well on the waveguide device is behaving as an appropriate control to be used to compare the cells exposed to NO. The two major limitations of this system are currently the expense and complexity of the device itself and the difficulty in imaging cells through the multiple layers of semi-opaque polymers used to &QJ;coat the bottom of the culture wells. The culture wells and &QJ;housing for the waveguide device are custom fabricated and are not disposable, making large numbers of replicates particularly labor intensive to collect.
Conclusions and future direction
We have developed a robust, solid core waveguide device that allows tunable levels of NO to be generated across the surface upon which cells can be cultured. The level of NO generated can be tuned by controlling the light used to illuminate the waveguide without exposing the cells to optical radiation. In this proof of concept system, we cultured MOVAS cells on a surface of SNAP–PDMS/gelatin and observed that cell growth was inhibited by the NO release levels and the cells in the control well without NO release were not visibly affected by the light used to release NO nor by NO from the neighboring well on the waveguide device. In addition to the gelatin used here, a number of different substrates could be adhered to the PDMS via the polydopamine layer, such as fibronectin, purified collagen materials, nanofibers or other polymer substrates that are intended to interact with cells. Due to the control of NO release from the SNAP–PDMS, the level and duration of release, as well timing of NO release can now be systematically studied for a wide variety of cells. The ability to include the temporal aspect of NO delivery to cells (i.e., continuous release from the time of seeding, release initiated after cells reach confluence, pulsed release, etc.) as a controllable variable to modulate is a powerful and essential aspect of understanding physiological and pathological effects of NO.
Acknowledgments
The authors are grateful for financial support from the National Science Foundation, Division of Materials Research DMR-0906709-2009.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Thomas D.D., Ridnour L.A., Isenbuerg J.S., Flores-Santana W., Switzer C.H., Donzelli S., Hussain P., Vecoli C., Paolocci N., Ambs S., Colton C.A., Harris C.C., Roberts D.D., Wink. D.A. The chemical biology of nitric oxide: implications in cellular signaling. Free Radical Biology and Medicine. 2008;45:18–31. doi: 10.1016/j.freeradbiomed.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaise G.A., Gauvin D., Gangal M., Authier. S. Nitric oxide, cell signaling and cell death. Toxicology. 2005;208:177–192. doi: 10.1016/j.tox.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 3.Li C.-Q., Wogon G.N. Nitric oxide as a modulator of apoptosis. Cancer Letters. 2005;226:1–15. doi: 10.1016/j.canlet.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Calabrese V., Mancuso C., Calvani M., Rizzarelli E., Butterfield D.A., Giuffrida Stella A.M. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nature Reviews Neuroscience. 2007;8:766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- 5.Vidwans A.S., Kim S., Coffin D.O., Wink D.A., Hewitt S.J. Analysis of the neuroprotective effects of various nitric oxide donor compounds in murine mixed cortical cell culture. Journal of Neurochemistry. 1999;5:1843–1852. doi: 10.1046/j.1471-4159.1999.0721843.x. [DOI] [PubMed] [Google Scholar]
- 6.Ridnour L.A., Thomas D.D., Switzer C., Flores-Santana W., Isenberg J.S., Ambs S., Roberts D.D., Wink. D.A. Molecular mechanisms for discrete nitric oxide levels in cancer. Nitric Oxide. 2008;19:73–76. doi: 10.1016/j.niox.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grisham M.B., Jourd’heuil D., Wink. D.A. Nitric oxide. I. physiological chemistry of nitric oxide and its metabolites: implications in inflammation. American Journal of Physiology – Gastrointestinal and Liver Physiology. 1999;276:G315–G321. doi: 10.1152/ajpgi.1999.276.2.G315. [DOI] [PubMed] [Google Scholar]
- 8.Vaughn M.W., Kuo L., Liao J.C. Estimation of nitric oxide production and reactionrates. AJP – Heart and Circulatory Physiology. 1998;274:H2163–H2176. doi: 10.1152/ajpheart.1998.274.6.H2163. [DOI] [PubMed] [Google Scholar]
- 9.Frost M.C., Reynolds M.M., Meyerhoff M.E. Polymers incorporating nitric oxide releasing/generating substances for improved biocompatiblity of blood-contacting medical devices. Biomaterials. 2005;26:1685–1693. doi: 10.1016/j.biomaterials.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds M.M., Frost M.C., Meyerhoff M.E. Nitric oxide-releasing hydrophobic polymers: preparation, characterization, and potential biomedical applications. Free Radical Biology and Medicine. 2004;37:926–936. doi: 10.1016/j.freeradbiomed.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 11.Keefer L.K. Progress toward clinical applications of the nitric oxide-releasing diazeniumdiolate. Annual Review of Pharmacology and Toxicology. 2003;43:585–607. doi: 10.1146/annurev.pharmtox.43.100901.135831. [DOI] [PubMed] [Google Scholar]
- 12.Hetrick E.M., Prichard H.L., Klitzman B., Schoenfisch M.H. Reduced foreign body response at nitric oxide-releasing subcutaneous implants. Biomaterials. 2008;28:4571–4580. doi: 10.1016/j.biomaterials.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frost M., Meyerhoff M.E. In vivo chemical sensors: tackling biocompatibility. Analytical Chemistry. 2006;78:7370–7377. doi: 10.1021/ac069475k. [DOI] [PubMed] [Google Scholar]
- 14.Shin J.S., Schoenfisch M.H. Improving the biocompatibility of in vivo sensors via nitric oxide release. The Analyst. 2006;131:609–615. doi: 10.1039/b600129g. [DOI] [PubMed] [Google Scholar]
- 15.Gierke G.E., Neilsen M., Frost M.C. S-Nitroso-N-acetyl-d-penicillamine covalently linked to polydimethylsiloxane (SNAP–PDMS) for use as a controlled photoinitiated nitric oxide release polymer. Science and Technology of Advanced Materials. 2011;12:055007. doi: 10.1088/1468-6996/12/5/055007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Starrett M.A., Nielsen M., Smeenge D.M., Romanowicz G.E., Frost M.C. Wireless platform for controlled nitric oxide releasing optical fibers for mediating biological response to implanted devices. Nitric Oxide. 2012;27:228–234. doi: 10.1016/j.niox.2012.08.074. [DOI] [PubMed] [Google Scholar]
- 17.Moynihan H.A., Robert S.M. Preparation of some novel S-nitroso compounds as potential slow-release agents of nitric oxide in vivo. Journal of the Chemical Society, Perkins Transaction. 1994;1:797–805. [Google Scholar]
- 18.Cornwell T.L., Arnold E., Boerth N.J., Lincoln T.M. Inhibition of smooth muscle cell growth by nitric oxide and activation of cAMP-dependent protein kinase by cGMP. American Journal of Physiology—Cell Physiology. 1994;267:C1405–C1413. doi: 10.1152/ajpcell.1994.267.5.C1405. [DOI] [PubMed] [Google Scholar]
- 19.Rudic R.D., Shesely E.G., Maeda N., Smithies O., Segal S.S., Sessa W.C. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. Journal of Clinical Investigation. 1998;108:731–736. doi: 10.1172/JCI1699. [DOI] [PMC free article] [PubMed] [Google Scholar]


