Figure 1.
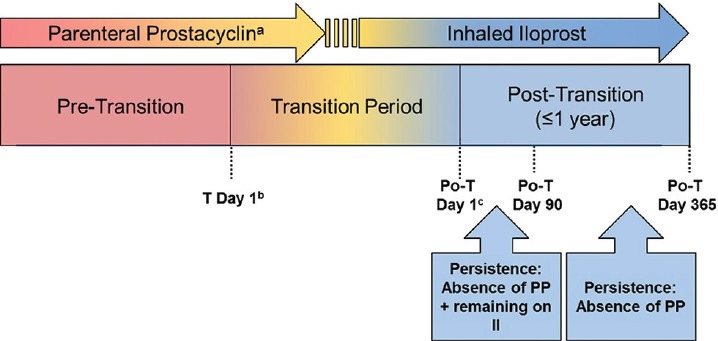
Study design. aParenteral prostacyclin comprises intravenous epoprostenol and intravenous/subcutaneous treprostinil. bTransition Day 1 is defined as the start day of inhaled iloprost with intent of discontinuing parenteral prostacyclin therapy. cPost-transition Day 1 is defined as the first day on inhaled iloprost and free of parenteral prostacyclin therapy. Depending on the clinical site and/or patient, there may be no period of concurrent (overlapping) administration of inhaled iloprost and parenteral prostacyclin therapy and, therefore, no transition period. In such cases, transition Day 1 is the same as post-transition Day 1. Po-T: post-transition; PP: parenteral prostacyclin; II: inhaled iloprost.
