Figure 4.
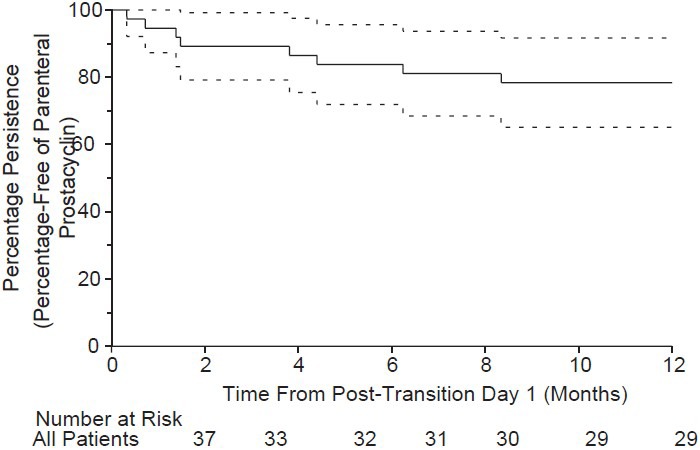
One-year estimates of persistence from post-transition Day 1 in all patients were as follows: iloprost monotherapy, 20.0 ± 17.9%; iloprost + ERA, 92.9 ± 6.9%; iloprost + PDE-5, 83.3 ± 10.8%; iloprost + ERA + PDE-5, 83.3 ± 15.2%. Log-rank P value, 0.009. ERA: endothelin receptor antagonist; PDE-5: phosphodiesterase type-5.
