Abstract
Introduction:
For orthodontists, the ideal bonding material should be less moisture-sensitive and should release fluoride, thereby reducing unfavorable iatrogenic decalcification. Resin-Modified Glass Ionomer Cements (RMGICs), due to their ability to bond in the presence of saliva and blood can be a very good bonding agent for orthodontic attachments especially in the areas of mouth, which are difficult to access. Moreover, their fluoride releasing property makes them an ideal bonding agent for patients with poor oral hygiene. However, their immediate bond strength is said to be too low to immediately ligate the initial wire, which could increase the total number of appointments. The effect of sandblasting and the use of sodium hypochlorite (NaOCL) on the immediate bond failure of RMGIC clinically have not been reported in the literature until the date. This investigation intended to assess the effect of sandblasting (of the bracket base and enamel) and NaOCL on the rate of bond failure (with immediate ligation at 30 min) of Fuji Ortho LC and its comparison with that of conventional light cured composite resin over a period of 1 year.
Materials and Methods:
400 sample teeth were further divided into 4 groups of 100 each and bonded as follows: (1) Group 1: Normal metallic brackets bonded with Fuji Ortho LC. (2) Group 2: Sandblasted bracket base and enamel surface, brackets bonded with Fuji Ortho LC. (3) Group 3: Deproteinized enamel surface using sodium hypochlorite and brackets bonded with Fuji Ortho LC. (4) Group 4: Normal metallic bracket bonded with Transbond XT after etching enamel with 37% phosphoric acid. This group served as control group.
Results and Conclusion:
Results showed that sandblasting the bracket base and enamel, can significantly reduce the bond failure rate of RMGIC.
Keywords: Bond failure, resin-modified glass ionomer cement, sandblasting
Introduction
Direct bonding of orthodontic brackets with composite resin provides greater comfort for patient, eliminates pre-treatment separation, decreases gingival irritation, improves oral hygiene and esthetics and reduces chairside time. Still, clinical improvements in orthodontic bonding are needed in two major areas - reduction of white spot lesions and increased tolerance to moisture contamination to reduce the incidence of bond failure.[1]
Glass ionomer cements first introduced for use in clinical restorative dentistry[2] have been shown to release fluoride over the long-term and at much higher levels than fluoride-releasing composites. However, they have poor bond strength, (2.37-5.5 MPa). Resin-modified glass ionomer cements (RMGICs), mimic glass ionomer cements with respect to fluoride release and fluoride recharging, but have widely varying bond strengths (5.39-18.9 MPa).[3]
The majority of failures involve cohesion within glass ionomer cement or adhesion involving the enamel. Composite resin bonding on the other hand, presents a mechanical risk to the enamel during debonding and exposure of the surface to scratching during the removal of excess adhesive. Glass ionomers can be scraped off with a curette with no adverse effects on the enamel.[4]
Orthodontists have been reluctant to use RMGIC as a routine bracket adhesive because of shear bond strength (SBS) issues despite many studies demonstrating comparable SBS with composite resin. Few in vivo studies[5,6] revealed that Transbond XT and Fuji Ortho LC had a comparable bond failure rate. Toledano et al.[7] concluded that SBS of RMGIs were acceptable only when phosphoric acid is used as an etchant. An ex vivo study[8] showed an increase in SBS with RMGI when enamel surface was etched and moistened with artificial saliva before bonding the bracket to the tooth. Roeder et al.[9] compared the composite bond strength with different enamel preparations such as air abraded only; air abraded plus adhesive; air abraded plus acid etch plus adhesive, and concluded that mean bond strength of the last group was the highest among the three groups. The bond strength of RMGIC was statistically less than that of Transbond XT both at 30 min and 24 h.
An unpublished study performed at Manipal College of Dental Sciences, Manipal concluded that sandblasting the bracket base alone and sandblasting the bracket base plus the enamel can increase the immediate bond strength of RMGIC to the level of resin based composite Transbond XT. Additional etching of the enamel after sandblasting seems to decrease the bond strength of RMGIC.
Espinosa et al.[10] showed that conditioning the enamel surface with 5.25% sodium hypochlorite (NaOCL) for 1 min, before acid etching, increased the quality of the etching pattern because NaOCl eliminated the organic matter from the enamel surface (deproteinization). Justus et al.[11] concluded from an in vitro study that with NaOCl use, bracket bond strength with Fuji Ortho LC is similar to Transbond XT.
Bishara et al.[12] concluded that RMGIC has significantly lower initial bond strength, but increased more than 20-fold within 24 h. In comparison, composite adhesive has a significantly larger initial bond strength that doubled within 24 h. The low initial bond strength of Fuji Ortho LC necessitates a second appointment for placing the arch wire; which means an increase in the total number of appointments made during the treatment and makes time management more difficult for the orthodontist.
The effect of sandblasting and the use of NaOCl on immediate bond-failure of RMGIC clinically has not been reported in literature until the date. So this investigation intended to assess the effect of sandblasting (of the bracket base and enamel) and NaOCl on the rate of bond-failure (with immediate ligation at 30 min) of Fuji Ortho LC and its comparison with that of conventional light-cured composite resin over a period of 1 year.
Materials and Methods
This study was approved by the Institutional Ethics Committee, Manipal University.
A total of 25 patients with mean age 22.16 years (age range = 15-32 years) participated in this study (16 teeth were bonded in each patient excluding the first premolars: 25 × 16 = 400 teeth). All had dental malocclusions and were accepted for fixed orthodontic treatment in the Department of Orthodontics and Dentofacial Orthopedics, Manipal College of Dental Sciences, Manipal. A consent form was obtained from each participant after explanation of the whole project. Patients with Class II division 2 malocclusion, deep bite, occlusal interferences or masochistic habits were excluded from the study. No bonded enamel surface presented caries, filling or hypoplasia.
Materials and Methods
A total of 400 sample teeth were further divided into 4 groups of 100 each.
To reduce the variables of an in vivo study, these 4 subgroups were sequentially allocated for the 4 quadrants of each patient such that every fifth patient had a similar sequence.
Bonding of brackets
The brackets used were 0.022 inch stainless steel Roth brackets (Gemini Series, 3M Unitek). All brackets were bonded in the middle third of the buccal surface of each tooth with the long axis of the bracket parallel to that of the tooth. All teeth were prepared by the same operator. Only two teeth were prepared and bonded at a time.
Group 1: Normal metallic brackets bonded with Fuji Ortho LC
Following procedure was followed for bonding of brackets with Fuji Ortho LC:
Prophylaxis was carried out using the pumice and teeth were rinsed thoroughly with water.
Sample tooth was not dried.
Fuji Ortho LC was mixed according to the manufacturer's instructions.
Bonding surface of the bracket was coated with cement without creating voids.
Bracket was positioned on the tooth, pressed firmly and excess cement was removed.
Curing was done for a total of 20 s (10 s on both mesial and distal side) using light emitting diode (LED).
Group 2: Bracket base and enamel were sandblasted and brackets bonded with Fuji ortho LC
Sandblasting was carried out with 50-micron aluminum oxide using intra-oral sandblaster (MICROETCHER™ ERC, DANVILLE MATERIALS, SAN RAMON, CA). The brackets were held with the help of reverse action bracket holders and the base of brackets sandblasted with 50-micron aluminum oxide for 5 s, at 60 psi pressure, with a nozzle distance of 10 mm and 45° angulation. After sandblasting the brackets, surfaces were cleaned with compressed air, to remove the sandblasting powder. Then, buccal enamel surfaces were sandblasted using the same procedure for 3 s. No etching of enamel was carried out for this group. The sandblasted brackets were bonded with Fuji Ortho LC using the same procedure as for Group 1.
Group 3: Enamel surface was deproteinized using NaOCL and brackets were bonded with Fuji Ortho LC
Following prophylaxis, deproteinization of the enamel surface was carried out with 5.25% NaOCl for 1 min using a microbrush.
This was followed by rinsing, drying and acid etching with 37% phosphoric acid for 30 s.
Subsequently, the acid was rinsed off and enamel was dried.
The brackets were then bonded with Fuji Ortho LC using the same procedure as for Group 1.
Group 4: Normal metallic brackets were bonded with Transbond XT after etching enamel with 37% phosphoric acid
This group served as a control group. The enamel surface was first cleaned with pumice and acid etched with 37% phosphoric acid for 30 s, thoroughly rinsed with water from a 3-way syringe for 30 s and then dried with an oil-free source for 20 s. Transbond primer was applied over the etched enamel surface and cured for 5 s. Then Transbond XT was applied over the bracket base, bracket was pressed firmly against the tooth, excess was removed and the composite was cured for 20 s.
After 30 min of bonding the brackets, a 0.014" stainless steel arch wire was ligated, which was sequentially changed to heavier wires as the treatment progressed.
To maintain consistency between the control sample and experimental sample in extraction and non-extraction cases, brackets on the first premolars were not included.
All patients were observed for 12 months during their regular orthodontic appointments. When a bracket debonded autonomously, it was recorded and another bracket was replaced using Transbond XT similar to Group 4 (control group). After failure, the tooth was no longer considered with respect to bond failure.
Scanning electron microscopy
Two representative specimens from each group (from patients included in the study requiring extractions) were left unbonded. The specimens were sputter coated with gold palladium and observed in a scanning electron microscope (SEM) (JEOL, JSM-6380 LA) at a magnification of 1600 times.
Method of statistical analysis
Frequency and the percentage was used to summarize failure rate across the four groups. Chi-square test was used as a test of significance to compare failure rates across the groups, arches, sides, type of teeth, time, and type of wire.
Results
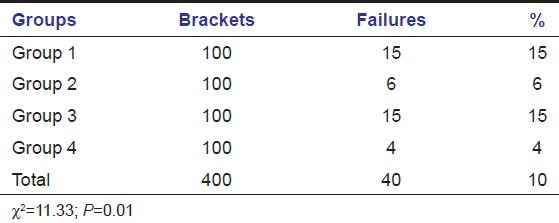
The bracket failures were recorded for each group over the first 12 months of active treatment. There were significant differences among the groups in terms of failure rates (P ≤ 0.01) [Table 1]. The overall failure rate for the sample (percentage of brackets requiring rebonding) was 10%. Highest failures (15%) occurred in Groups 1 and 3; six (6%) failures occurred with Group 2 and lowest failures (4%) occurred with the control Group 4.
Table 1.
Number, frequency of failures, and statistical comparison between composite and resin-modified glass ionomer cement groups
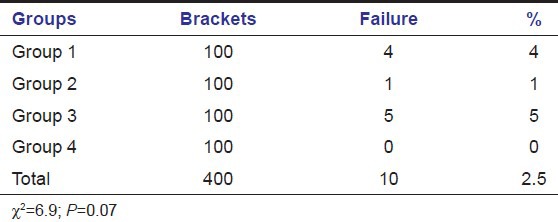
Immediate failures were highest in Group 3 (5%) followed by Group 1 (4%) and Group 2 (1%). There were no immediate failures in the control Group 4 [Table 2].
Table 2.
Immediate bond failure among groups
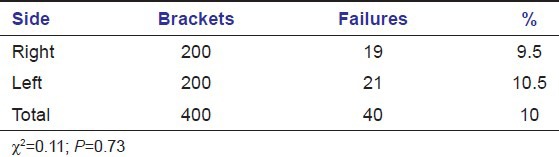
There was no significant difference in failure rate depending on arch (maxilla, mandible) or side (right, left) of failure [Tables 3 and 4].
Table 3.
Failure rate by arch location
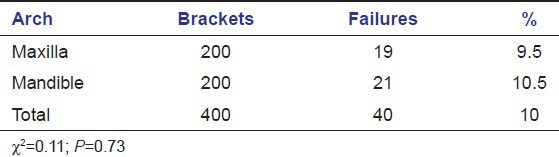
Table 4.
Failure rate by side
Data relating to the location of brackets were grouped into 4 categories depending on tooth type: Central incisors, lateral incisors, canines, and premolars. There were significant differences according to location in terms of the failure rates (P ≤ 0.01). The failure rates were higher for premolars (18% of the brackets failed) compared to lateral incisors (11% failed), central incisors (6% failed) and canines (5% failed). The failure rate of brackets was lowest for the canines [Table 5].
Table 5.
Failure rate by location of bracket
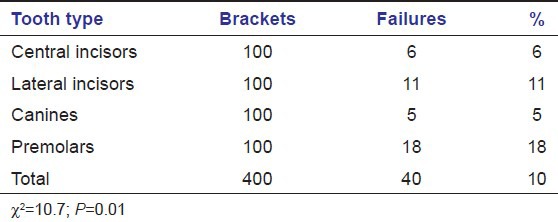
Nearly, 2.5% of the brackets failed immediately. The highest failure rate was seen in first 4 months (5.4%) and lowest failure rate (0.8%) was observed in last 4 months of the study [Table 6].
Table 6.
Failure rate with respect to time
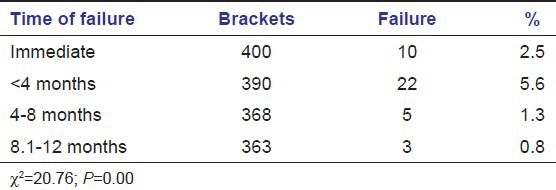
Round leveling wires showed more failures (7.7%) as compared to the rectangular wires used later during the treatment (2.5%) [Table 7].
Table 7.
Failure rate by type of wire
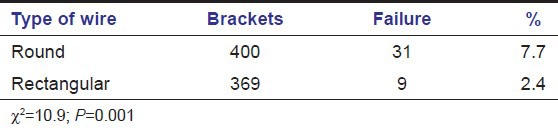
SEM results
SEM images of enamel surface morphologies after surface pre-treatment of various groups have been shown [Figures 1-4]. The untreated enamel (Group 1) showed a smooth surface [Figure 1]. Sandblasting (Group 2) produced a less well defined pattern on the enamel with irregular grooving of the enamel surface [Figure 2]. The enamel preconditioned with NaOCl (Group 3) produced a qualitatively rougher enamel surface [Figure 3] showing better etch pattern. Acid etching (Group 4) produced a very subtle, uneven surface topography [Figure 4].
Figure 1.
Scanning electron microscope image for untreated enamel surface (Group 1)
Figure 4.
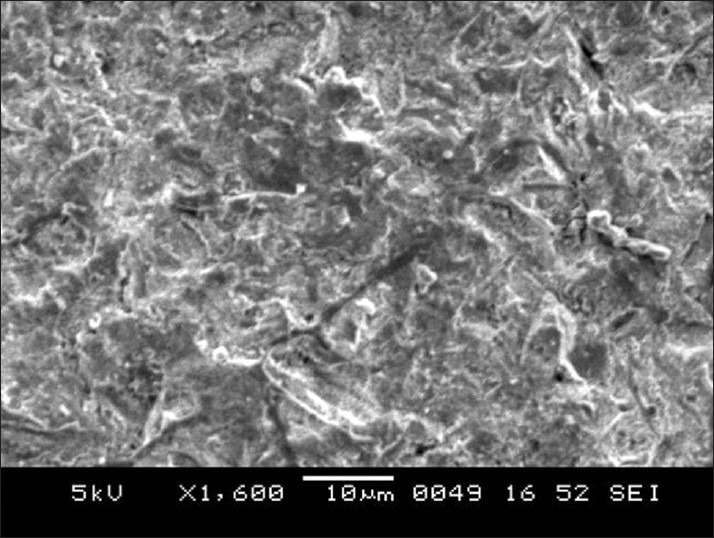
Scanning electron microscope image for acid etched enamel surface (Group 4)
Figure 2.

Scanning electron microscope image for sandblasted enamel surface (Group 2)
Figure 3.
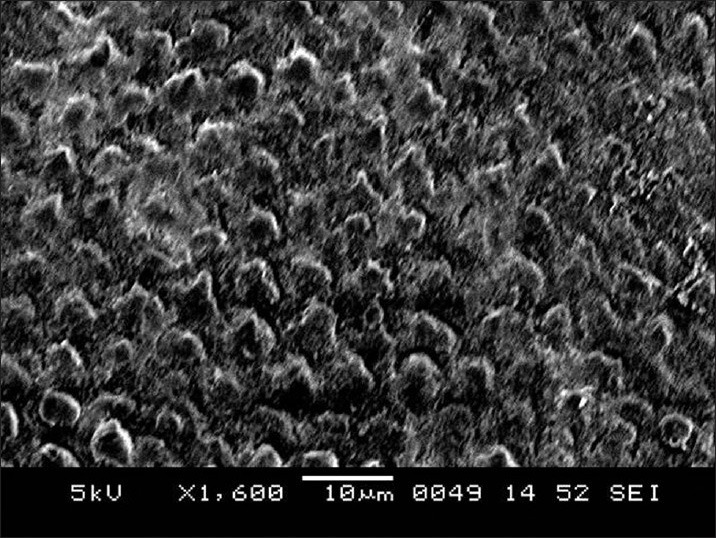
Scanning electron microscope image for enamel surface pre-conditioned with sodium hypochlorite and acid etched (Group 3)
Discussion
Orthodontic brackets are routinely bonded to enamel using the acid-etch technique in conjunction with a composite-type orthodontic adhesive. The glass ionomer cements were developed to aggregate the adhesive, biological and chemical properties into one material. Such cements not only provide enamel bonding, but also release and reload fluoride so that white spot lesions can be reduced. In addition, this material facilitates the debonding procedures.
Most contemporary glass ionomer adhesives used for bonding orthodontic attachments have tricure setting mechanism, which has resulted in bond strength, which is less, but clinically acceptable as compared to conventional resin based composite adhesive at 24 h.[1,13,14,15,16,17,18,19,20,21]
Some studies have documented that resin based adhesives have sufficient bond strength to withstand arch wire ligation soon after bonding, but the immediate bond strength for RMGIC is far less than clinically acceptable limits.[12,15,22] This could result in either immediate de-bonding or the orthodontist has to wait for at least 24 h so that the adhesive could attain sufficient bond strength.
The previous literature does not provide any defined protocol for sandblasting the enamel and base of the bracket. In an in vitro study Ozer and Arici[23] used 25 μm aluminum oxide particle for 3 s at 58 psi pressure with micro-etcher held at 30 mm of distance with 90° angle to the bracket base, whereas Sunna and Rock[24] in an in vivo study used 90 μm aluminum oxide particle for 3 s at 58 psi pressure with micro-etcher held at 40 mm distance. Millett et al.[25] carried out sandblasting of bracket base at 10 mm distance for 3 s. Reisner et al.[26] sandblasted the buccal surfaces of the premolars at 65-70 psi pressure for 2-3 s with 50 μm aluminum oxide particle. In the present investigation, a uniform 50 μm aluminum oxide particle at pressure of 60 psi at a distance of 10 mm was used for sandblasting both enamel and metallic bracket base. However, the duration of sandblasting was 5 s for metal bracket base and 3 s for enamel. Longer duration was used for bracket base to compensate for reduced size of the aluminum oxide particle.
Sand-trap in the form of soft plastic spheres slipping onto the suction with the micro-etcher tip coming through the top opening was used to trap the abrasive and prevent any health hazards to patients. In addition, sand-trap helped in maintaining a constant distance of 10 mm between the tooth and micro-etcher tip.
In the present study, LED curing unit was used to cure Transbond XT and RMGIC. Use of LED curing light not only reduces curing time from 40 s (conventional halogen lights) to 20 s without compromising the bond strength at any light tip distance,[22] it also induces significantly less increase in intrapulpal temperature than conventional halogen lights and Xenon Plasma Arch.[27]
LED curing unit also results in higher bond strength as compared to conventional halogen curing light.[28]
Once Fuji Ortho LC liquid/powder has been mixed, the operator has less than a minute or two (depending on room temperature) to position the brackets before the adhesive begins to harden. This probably occurs due to the ambient light. Therefore, in the present study adhesive was prepared for only 2 teeth at a time. Even sandblasting and NaOCl application was carried out for 2 teeth at a time. The saliva suction tip was positioned in such a fashion as to suction away all NaOCl excess.
It was recommended that clinical studies evaluating bond-failure rates should either record only first-time failures or analyze multiple failures of the same site in a different category because the failure rate of the second and third-time bonds are observed more frequently compared with first-time.[29] Hence, only first-time bond failures were evaluated in this study.
In the present study, the overall failure rate for the sample (percentage of brackets requiring rebonding) was 10%. Highest failure (15%) was recorded for Groups 1 and 3. There is a controversy in the literature regarding the clinical performance of RMGIC as a bonding material. Few studies[6,14] showed no significant difference in bracket failure rates between Fuji Ortho LC and composite resin. Some studies[16,30] showed that bonding brackets and molar tubes with Fuji Ortho LC was compatible with clinical orthodontic practice. However, our results were in accordance with Larmour and Stirrups[31] and Hegarty and Macfarlane[32] who suggested that in the clinical situation the use of Fuji Ortho LC may result in unacceptable bond failure rates. It should be noted that observation periods, materials (brackets, adhesives) and enamel surface conditioning widely differ from one study to another.
In the present study, deproteinized enamel was further acid etched before bonding with Fuji Ortho LC as recommended by Justus et al.[11] Our results showed a high failure rate (15%) in this group, which was in contrast with Justus et al.[11] who concluded from an in vitro study that with NaOCl use, bracket bond strength with Fuji Ortho LC became similar to Transbond XT. This can be explained by the fact that liquid to powder ratio of RMGIC is 3:1, which results in a very thick mix and it is difficult for this thick adhesive to penetrate the fine etching pattern (2-5 μm in diameter) produced by acid etching [Figure 3]. Moreover, etching also reduces effective number of Ca++ ions, which are available from the enamel to get bonded with the COO− group of polycarboxylic acid, which is mainly responsible for the final 24-h bond strength of RMGIC. Therefore, the authors recommend further clinical studies to evaluate the performance of Fuji Ortho LC on deproteinized enamel without acid etching.
An interesting finding of this study was a significantly low failure rate (6%) in Group 2 which was comparable to control group 4 (4%). The reduced bond-failure rate can be explained by an increase in surface roughness produced by sandblasting the bracket base and enamel.[23,33] Reisner et al.[26] reported roughness of prepared enamel produced by sandblasting to be similar to that of acid etching. Increased roughness of bracket base results in more retentive areas available at the mesh base, which could be engaged by RMGIC. Sandblasting enamel not only increases the roughness of enamel, it also results in an increase in the number of exposed Ca++ (from enamel), which are available for chelation by carboxyl group of polycarboxylic acid present in the liquid of RMGIC.[34]
Sandblasting produced a less well-defined pattern on the enamel with grooving of the enamel surface [Figure 2]. Obtuse angularities produced by sandblasting are shallow and wide and RMGIC can easily get adapted to the irregularities especially in the presence of moisture.
The control group showed the lowest bond-failure rate (4%) in this study. This can be explained by the fact that acid etching produces a well-defined etching pattern by preferential dissolution of either periphery or core of the enamel. The bonding agent used with conventional composite has a very low viscosity and very high surface energy, which helps it to easily penetrate the irregularities of the etching pattern and produce resinous tags.
A very subtle, uneven surface topography was created for SEM images taken for this group [Figure 4] because dissolution rate in acids differs for various parts of the enamel structure, particularly between interprismatic and prismatic enamel. This is in accordance with the observations by Mattick and Hobson[35] in that an ideal etch pattern is found in only 5% of etched enamel on the buccal surfaces of teeth and Hobson and McCabe et al.[36] who concluded that high bond strength does not depend on an ideal etch pattern.
Immediate failures were highest in Group 3 (5%) followed by Group 1 (4%) and Group 2 (1%) thus showing that sandblasting can come handy for utilization of RMGIC as a bonding material.
There was no significant difference in failure rate depending on arch or side of failure. This could be due to smaller sample size in the study.
The highest failure rate was seen in the premolar region (18%). This could be because of high occlusal forces in the posterior region and variable contour of the posterior teeth.
Round wires were associated with significantly more failures than rectangular wires. This was because during the initial 4 months when maximum failures occurred round wires were used for leveling. The significantly lower failure rate with rectangular wires could be explained by the fact that rectangular wires were used once the teeth were leveled out and crowding was relieved. However, it can also be stated that the increased wire dimension did not lead to a higher bracket failure.
Since, it was a pilot study, a smaller sample size was taken and a further study with a larger sample size is recommended before justifying routine clinical use of the procedures used in this study to reduce the clinical failure of RMGICs.
Conclusions
The bond failure rate of RMGIC on unprepared and deproteinized enamel was significantly high than that of Transbond XT.
Sandblasting the bracket base plus sandblasting the enamel can reduce the immediate and long term bond-failure rate of RMGIC.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Bishara SE, Olsen ME, Damon P, Jakobsen JR. Evaluation of a new light-cured orthodontic bonding adhesive. Am J Orthod Dentofacial Orthop. 1998;114:80–7. doi: 10.1016/s0889-5406(98)70242-2. [DOI] [PubMed] [Google Scholar]
- 2.Wilson AD, Kent BE. A new translucent cement for dentistry. The glass ionomer cement. Br Dent J. 1972;132:133–5. doi: 10.1038/sj.bdj.4802810. [DOI] [PubMed] [Google Scholar]
- 3.Rix D, Foley TF, Mamandras A. Comparison of bond strength of three adhesives: Composite resin, hybrid GIC, and glass-filled GIC. Am J Orthod Dentofacial Orthop. 2001;119:36–42. doi: 10.1067/mod.2001.110519. [DOI] [PubMed] [Google Scholar]
- 4.Klockowski R, Davis EL, Joynt RB, Wieczkowski G, Jr, MacDonald A. Bond strength and durability of glass ionomer cements used as bonding agents in the placement of orthodontic brackets. Am J Orthod Dentofacial Orthop. 1989;96:60–4. doi: 10.1016/0889-5406(89)90230-8. [DOI] [PubMed] [Google Scholar]
- 5.Koyal S, Valiathan A. Comparison of bond failure of Fuji Ortho LC Transbond XT A clinical study. J Pierre Fauchard Acad. 2003;17:17–25. [Google Scholar]
- 6.Summers A, Kao E, Gilmore J, Gunel E, Ngan P. Comparison of bond strength between a conventional resin adhesive and a resin-modified glass ionomer adhesive: An in vitro and in vivo study. Am J Orthod Dentofacial Orthop. 2004;126:200–6. doi: 10.1016/j.ajodo.2003.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Toledano M, Osorio R, Osorio E, Romeo A, de la Higuera B, García-Godoy F. Bond strength of orthodontic brackets using different light and self-curing cements. Angle Orthod. 2003;73:56–63. doi: 10.1043/0003-3219(2003)073<0056:BSOOBU>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez J. master thesis. Mexico City, Mexico: Intercontinental University; 1997. Bond strength of a glass ionomer cement and a light cured composite: An ex vivo study. [Google Scholar]
- 9.Roeder LB, Berry EA, 3rd, You C, Powers JM. Bond strength of composite to air-abraded enamel and dentin. Oper Dent. 1995;20:186–90. [PubMed] [Google Scholar]
- 10.Espinosa R, Valencia R, Uribe M, Ceja I, Saadia M. Enamel deproteinization and its effect on acid etching: An in vitro study. J Clin Pediatr Dent. 2008;33:13–9. doi: 10.17796/jcpd.33.1.ng5462w5746j766p. [DOI] [PubMed] [Google Scholar]
- 11.Justus R, Cubero T, Ondarza R, Morales F. Fluoride-releasing resin-modified glass ionomer cements: Comparing shear bond strength of two adhesive systems with enamel surface deproteinization before etching. Semin Orthod. 2010;16:66–75. [Google Scholar]
- 12.Bishara SE, VonWald L, Olsen ME, Laffoon JF. Effect of time on the shear bond strength of glass ionomer and composite orthodontic adhesives. Am J Orthod Dentofacial Orthop. 1999;116:616–20. doi: 10.1016/s0889-5406(99)70195-2. [DOI] [PubMed] [Google Scholar]
- 13.Cacciafesta V, Bosch C, Melsen B. Clinical comparison between a resin-reinforced self-cured glass ionomer cement and a composite resin for direct bonding of orthodontic brackets Part 1: Wetting with water. Clin Orthod Res. 1998;1:29–36. doi: 10.1111/ocr.1998.1.1.29. [DOI] [PubMed] [Google Scholar]
- 14.Fowler PV. A twelve-month clinical trial comparing the bracket failure rates of light-cured resin-modified glass-ionomer adhesive and acid-etch chemical-cured composite. Aust Orthod J. 1998;15:186–90. [PubMed] [Google Scholar]
- 15.Bishara SE, VonWald L, Olsen ME, Laffoon JF, Jakobsen JR. Effect of light-cure time on the initial shear bond strength of a glass-ionomer adhesive. Am J Orthod Dentofacial Orthop. 2000;117:164–8. doi: 10.1016/s0889-5406(00)70227-7. [DOI] [PubMed] [Google Scholar]
- 16.Hitmi L, Muller C, Mujajic M, Attal JP. An 18-month clinical study of bond failures with resin-modified glass ionomer cement in orthodontic practice. Am J Orthod Dentofacial Orthop. 2001;120:406–15. doi: 10.1067/mod.2001.115931. [DOI] [PubMed] [Google Scholar]
- 17.Arici S, Arici N. Effects of thermocycling on the bond strength of a resin-modified glass ionomer cement: An in vitro comparative study. Angle Orthod. 2003;73:692–6. doi: 10.1043/0003-3219(2003)073<0692:EOTOTB>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.Oliveira SR, Rosenbach G, Brunhard IH, Almeida MA, Chevitarese O. A clinical study of glass ionomer cement. Eur J Orthod. 2004;26:185–9. doi: 10.1093/ejo/26.2.185. [DOI] [PubMed] [Google Scholar]
- 19.Godoy-Bezerra J, Vieira S, Oliveira JH, Lara F. Shear bond strength of resin-modified glass ionomer cement with saliva present and different enamel pretreatments. Angle Orthod. 2006;76:470–4. doi: 10.1043/0003-3219(2006)076[0470:SBSORG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Al Shamsi A, Cunningham JL, Lamey PJ, Lynch E. Shear bond strength and residual adhesive after orthodontic bracket debonding. Angle Orthod. 2006;76:694–9. doi: 10.1043/0003-3219(2006)076[0694:SBSARA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 21.Sfondrini MF, Cacciafesta V, Scribante A, Boehme A, Jost-Brinkmann PG. Effect of light-tip distance on the shear bond strengths of resin-modified glass ionomer cured with high-intensity halogen, light-emitting diode, and plasma arc lights. Am J Orthod Dentofacial Orthop. 2006;129:541–6. doi: 10.1016/j.ajodo.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 22.Bishara SE, Soliman M, Laffoon JF, Warren J. Shear bond strength of a new high fluoride release glass ionomer adhesive. Angle Orthod. 2008;78:125–8. doi: 10.2319/100405-347.1. [DOI] [PubMed] [Google Scholar]
- 23.Ozer M, Arici S. Sandblasted metal brackets bonded with resin-modified glass ionomer cement in vivo. Angle Orthod. 2005;75:406–9. doi: 10.1043/0003-3219(2005)75[406:SMBBWR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Sunna S, Rock WP. Effect of sandblasting on the retention of orthodontic brackets: A controlled clinical trial. J Orthod. 2008;35:43–8. doi: 10.1179/146531207225022410. [DOI] [PubMed] [Google Scholar]
- 25.Millett D, McCabe JF, Gordon PH. The role of sandblasting on the retention of metallic brackets applied with glass ionomer cement. Br J Orthod. 1993;20:117–22. doi: 10.1179/bjo.20.2.117. [DOI] [PubMed] [Google Scholar]
- 26.Reisner KR, Levitt HL, Mante F. Enamel preparation for orthodontic bonding: A comparison between the use of a sandblaster and current techniques. Am J Orthod Dentofacial Orthop. 1997;111:366–73. doi: 10.1016/s0889-5406(97)80018-2. [DOI] [PubMed] [Google Scholar]
- 27.Uzel A, Buyukyilmaz T, Kayalioglu M, Uzel I. Temperature rise during orthodontic bonding with various light-curing units: An in vitro study. Angle Orthod. 2006;76:330–4. doi: 10.1043/0003-3219(2006)076[0330:TRDOBW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Wendl B, Droschl H. A comparative in vitro study of the strength of directly bonded brackets using different curing techniques. Eur J Orthod. 2004;26:535–44. doi: 10.1093/ejo/26.5.535. [DOI] [PubMed] [Google Scholar]
- 29.Kinch AP, Taylor H, Warltier R, Oliver RG, Newcombe RG. A clinical trial comparing the failure rates of directly bonded brackets using etch times of 15 or 60 seconds. Am J Orthod Dentofacial Orthop. 1988;94:476–83. doi: 10.1016/0889-5406(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 30.Choo SC, Ireland AJ, Sherriff M. An in vivo investigation into the use of resin-modified glass poly (alkenote) cements as orthodontic bonding agents. Eur J Orthod. 2001;23:403–9. doi: 10.1093/ejo/23.4.403. [DOI] [PubMed] [Google Scholar]
- 31.Larmour CJ, Stirrups DR. An ex vivo assessment of a resin-modified glass ionomer cement in relation to bonding technique. J Orthod. 2001;28:207–10. doi: 10.1093/ortho/28.3.207. [DOI] [PubMed] [Google Scholar]
- 32.Hegarty DJ, Macfarlane TV. In vivo bracket retention comparison of a resin-modified glass ionomer cement and a resin-based bracket adhesive system after a year. Am J Orthod Dentofacial Orthop. 2002;121:496–501. doi: 10.1067/mod.2002.122367. [DOI] [PubMed] [Google Scholar]
- 33.Millett DT, McCabe JF. Orthodontic bonding with glass ionomer cement: A review. Eur J Orthod. 1996;18:385–99. doi: 10.1093/ejo/18.4.385. [DOI] [PubMed] [Google Scholar]
- 34.Sargison AE, McCabe JF, Millett DT. A laboratory investigation to compare enamel preparation by sandblasting or acid etching prior to bracket bonding. Br J Orthod. 1999;26:141–6. doi: 10.1093/ortho/26.2.141. [DOI] [PubMed] [Google Scholar]
- 35.Mattick CR, Hobson RS. A comparative micro-topographic study of the buccal enamel of different tooth types. J Orthod. 2000;27:143–8. doi: 10.1093/ortho/27.2.143. [DOI] [PubMed] [Google Scholar]
- 36.Hobson RS, McCabe JF. Relationship between enamel etch characteristics and resin-enamel bond strength. Br Dent J. 2002;192:463–8. doi: 10.1038/sj.bdj.4801401. [DOI] [PubMed] [Google Scholar]


