Figure 1.
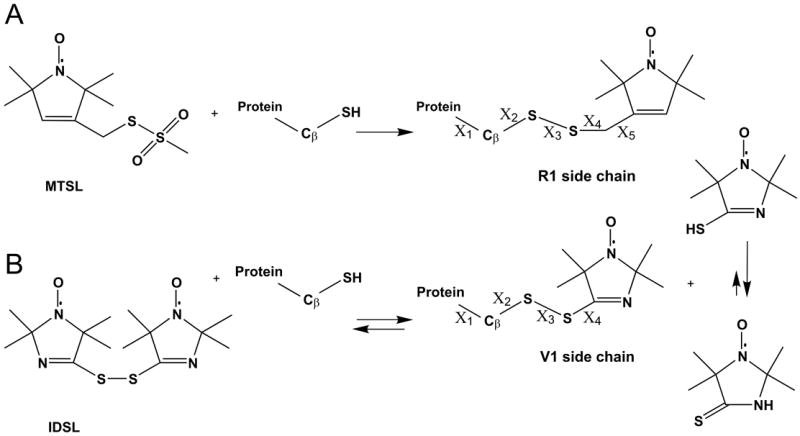
Introduction of nitroxide side chains via cysteine substitution mutagenesis. In each case, a cysteine residue is introduced at the site of interest, followed by reaction with the desired sulfhydryl specific reagent. (A), reaction with 1-Oxyl-2,2,5,5-tetramethypyrroline-3-methyl (MTSL) to generate R1. (B), reaction with bis(2,2,5,5-tetramethyl-3-imidazoline-1-oxyl-4-il)-disulfide (IDSL) to generate V1. Numbering of dihedral angles (X) is shown for each side chain.
