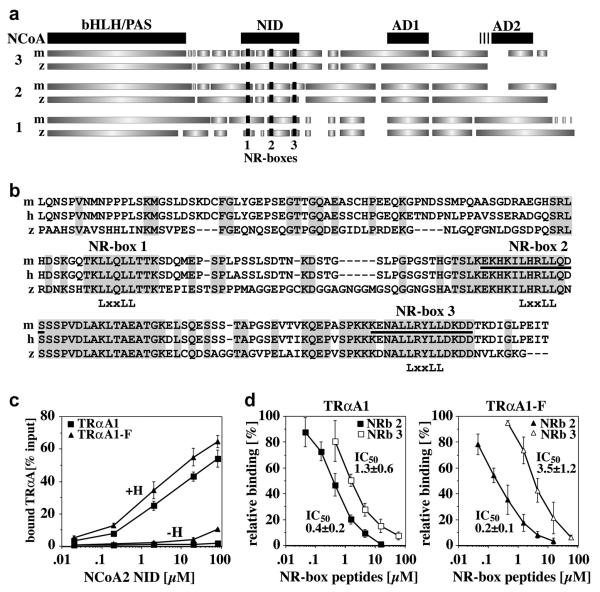
Fig. 4. The F-domain regulates the interaction of TRαA with the zebrafish coactivator NCoA2.
A, Domain structure of mouse and zebrafish NCoA1-3 based on a ClustalW sequence alignment (pHLH/PAS, helix-loop-helix/Per-Arnt-Sim domain; NID, nuclear receptor interaction domain; AD1 and AD2, activation domains 1 and 2. GeneBank accession numbers are: mNCoA3, NP_032705; zNCoA3, XP_692938 (predicted); mNCoA2, AAC53151; zNCoA2, AAK11608; mNCoA1, NM_010881; zNCoA1, CAI21172 (predicted). The nuclear receptor binding sites (NR-boxes 1-3) are shown in black. B, ClustalW alignment of the cloned zNCoA2 NID (aa 559-758) and the NIDs of mouse (m) and human (h) NCoA2 (both aa 563-764). The conserved “LxxLL” motifs of NR-boxes 1-3 are identified. Identical residues are grey shaded. The sequences of the NRb2 and NRb3 peptides used in (D) are underlined. C, Interaction of GST-bound zfNCoA2 NID (0.002-80 μM) with in vitro expressed, 35S-labeled TRαA1 and TRαA1-F in the presence of either DMSO (−H) or 10 μM T3 (+H). Shown are the averages and standard deviations of four binding experiments. The differences in the binding of TRαA1 and TRαA1-F at NCoA2 NID concentrations higher than 0.1 μM (+H) or higher than 10 μM (−H) are statistically significant (student’s t test p≤0.06). D, Competition of GST-bound zfNCoA2 NID (12 μM) and mNCoA2 NR-box 2 and 3 peptides (NRb 2, NRb 3) for binding to zebrafish TRαA1 and TRαA1-F. Peptides sequences are identified in (B) (underlined). Shown are the averages and standard deviations of four binding experiments. IC50 values are given in [μM].