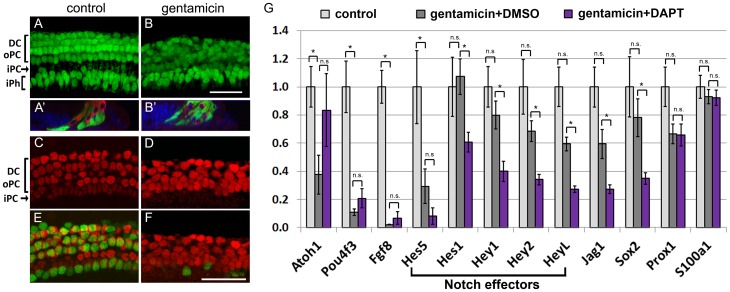
Figure 2. Supporting cell phenotype in the hair cell damaged cochlea.
A–B': P2 Lfng/GFP transgenic control (A, A') and gentamicin treated (B, B') cochlear cultures after 1 DIV. Deiters cell (DC), outer pillar cell (oPC) and inner phalangeal cell nuclei (iPh) are visualized by native Lfng/GFP expression (green); hair cells are immuno-stained with myosin VI antibody (Myo6, red). A, B: Confocal images of supporting cell layer. A' B': orthogonal section of z-stack projections of supporting cell and hair cell layer. 24 hours after initial gentamicin insult, Lfng/GFP positive supporting cell layer is disorganized (B) and myosin VI positive hair cell debris (red) is engulfed by Lfng/GFP positive supporting cells (green) (B'). DAPI (blue) labels cell nuclei. Scale bar 50 μm. C–F: Confocal images of P2 Atoh1/nGFP transgenic control (C, E) and gentamicin treated (D, F) cultures after 2 DIV. Prox1 immuno-staining (red) marks Deiters cells (DC), outer pillar cells (oPC) and inner pillar cell (iPC) nuclei and Atoh1/nGFP expression (green) marks hair cell nuclei. Distinct oval nuclear morphology of inner pillar cells seen in control cultures (C) is largely lost in gentamicin treated cultures (D). Scale bar: 50 μm. G: Relative mRNA levels of Notch effector genes (bracket) and Notch target genes (Jag1, Sox2) were analyzed in undamaged (control, white bar), hair cell damaged (gentamicin +DMSO, grey bar) and hair cell damaged and Notch inhibited (gentamicin +DAPT, purple bar) cochlear epithelia after 38 hours in culture using q-PCR. Gentamicin was added at plating and washed out after 14 hours of culture. DAPT or vehicle control DMSO was added following gentamicin treatment for 24 hours. Hair cell specific genes (Atoh1, Pou4f3, and Fgf8) and supporting cell specific genes (Prox1, S100a1) were analyzed as experimental controls. Data expressed as mean ±SEM (n = 5, independent experiments analyzed * p≤0.05, Student's t-test, p>0.05 was considered not significant (n.s)).