Figure 1.
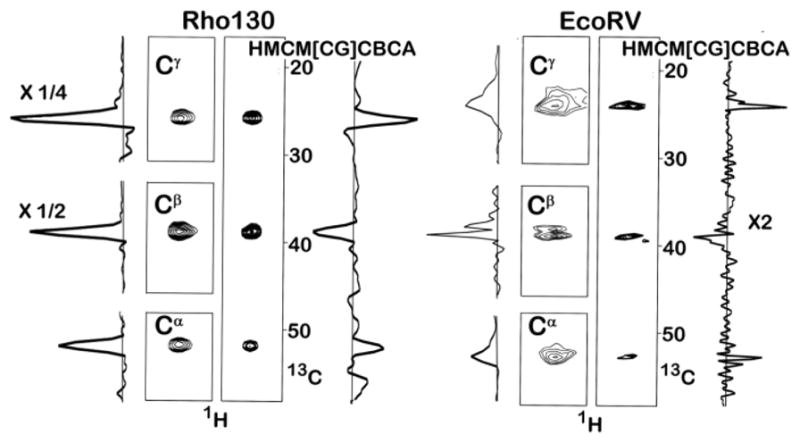
Leucine Methyl Correlation Spectra of Rho130 and the EcoRV-DNA Complex. The methyl carbon region was sampled using 21 complex points in all experiments. The Cα resonances are broadened by unresolved coupling to the 15N amide nitrogen. The concentration of Rho130 was 1.0 mM and spectra were acquired at 30°C. In the case of the Rho130 sample, all four spectra were acquired under identical condition; 8 scans with the same spectral width (50 ppm) and number of points (40) in the non-methyl carbon dimension. The total acquisition time/experiment was 9 hours. In the rho130 spectra the one dimensional trace for the Cβ peaks are divided by two and the trace for the Cγ peak was divided by four. The concentration of the EcoRV-DNA complex was 0.8 mM and the spectra were acquired at 35°C. In the case of EcoRV-DNA sample the spectral widths for the aliphatic carbons in the Cα Cβ, Cγ, and the HMCM[CG]CBCA experiments were 20 ppm, 60 ppm, 20 ppm, and 50 ppm, respectively. These were sampled using 32, 40, 32, and 40 complex points, respectively. A total of 40, 16, 8, and 40 scans were acquired for the Cα, Cβ, Cγ, and HMCM[CG]CBCA experiments, respectively, giving total acquisition times of 35.8, 17.92, 7.17, and 44.8 hours, respectively. The entire one dimensional trace for the HMCM[CG]CBCA experiment is multiplied by two. The increase in signal intensity for one residue from the EcoRV-DNA sample, after scaling for the total experimental time, is 2.0, 8.8, and 6.0 for the Cα, Cβ, Cγ, experiments. These ratios are the lower limit because many correlations could not be observed in the HMCM[CG]CBCA experiment. The Cβ experiment could have been run in ½ the time (8 scans), which would have given a total acquisition time for all three experiments of approximately 52 hours, i.e. not much longer than the entire HMCM[CG]CBCA experiment. Pulse sequences can be found in figure S1, along with additional guidelines for defining the acquisition time for the detection of non-methyl carbons.
