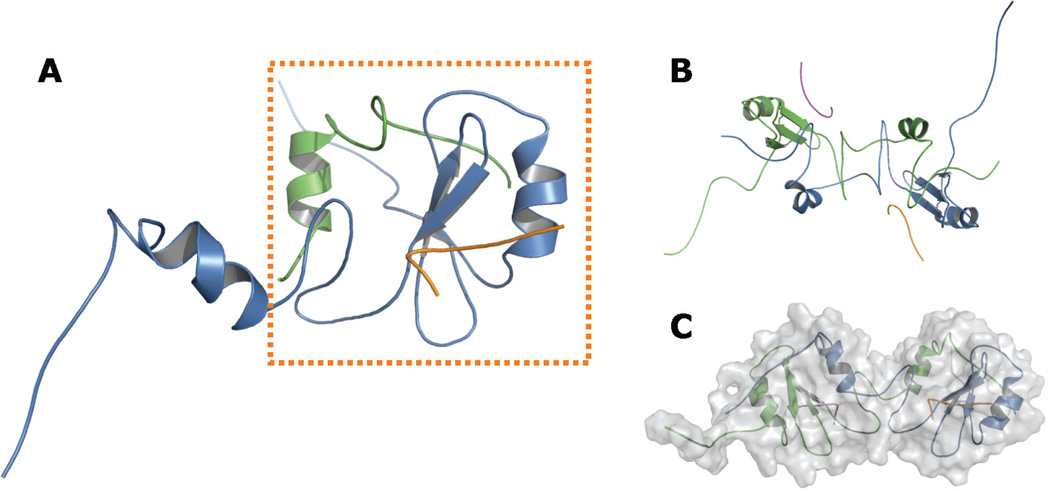
FIGURE 3.
Proposed helix-swapped Grb7-SH2 domain model. Panels A to C show various views of the Grb7-SH2 domain / pY1139 peptide complex following refinement using NMR-derived restraints. Each monomer and each ligand is shown in a distinct color. A, Side view of a single hybrid Grb7-SH2 domain “functional monomer,” shown in the dashed box. The N-terminal segment of the functional monomer is composed of approximately 85 residues from a single Grb7-SH2 domain chain (blue), ending with the unique Grb7-SH2 domain sequence MD-DGQ. The C-terminal segment is made up of approximately 33 residues from a second Grb7-SH2 domain chain (green), beginning with the sequence T’R’F’T’, which immediately follows M’D’D’G’Q’ in the second chain. The remaining residues of the chain are hidden for clarity. B, View along the axis of symmetry. The model shows C2 symmetry, although no symmetry was imposed in the refinement protocol. C, “Head-to-head” orientation of the two functional monomers, with the molecular surface shaded in gray. Figures rendered with PyMOL.36