Figure 1.
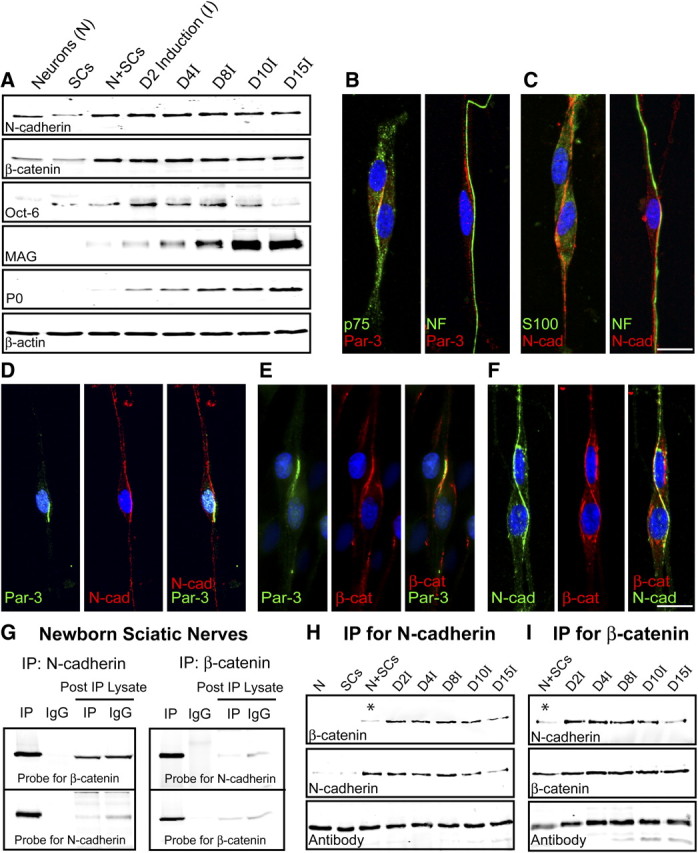
Expression and colocalization of Par-3 and N-cadherin in SCs. A, Western blot analysis of purified DRG neurons, SCs, and SC–DRG cocultures before (N+SCs) and after induction of myelination (denoted by I). Westerns blots were probed for N-cadherin, Oct-6, a transcription factor expressed by premyelinating SCs, and myelin proteins (MAG and P0). β-Actin serves as a loading control. B, The polarity protein Par-3 (red) is asymmetrically localized on SC–SC and SC–axon contact. p75NTR (green; first panel) is counterstained to identify the SCs and NF (green; second panel) for the axon. C, Similar to Par-3, N-cadherin (red) is asymmetrically localized on SC–SC and SC–axon contact. S100 (green; first panel) is counterstained to identify the SCs and NF (green; second panel) for the axon. The cell nuclei are detected by DAPI (blue). D, Par-3 and N-cadherin colocalize to the SC–axon interface in premyelinating SC–DRG cocultures. Par-3 (green) and N-cadherin (red) display asymmetric localization, and the merged image (third panel) displays colocalization (yellow). E, Par-3 and β-catenin colocalize to the SC–axon interface in premyelinating SC–DRG cocultures. Par-3 (green) and β-catenin (red) display asymmetric localization, and the merged image (third panel) displays colocalization (yellow). F, N-cadherin and β-catenin colocalize to the SC–axon interface in premyelinating SC–DRG cocultures. N-cadherin (green) and β-catenin (red) display asymmetric localization, and the merged image (third panel) displays colocalization (yellow). The nucleus is detected by DAPI (blue). Scale bars, 10 μm. G, N-cadherin and β-catenin coimmunoprecipitate in newborn rat sciatic nerves. β-Catenin coimmunoprecipitated with an antibody to N-cadherin and was detected by immunoblotting with an antibody to β-catenin. N-cadherin coimmunoprecipitated with an antibody to β-catenin and was detected by immunoblotting with an antibody to N-cadherin. Normal IgGs were used as a control for the immunoprecipitation. The blots were stripped and reprobed with the antibody for each of the respective targets. The post-immunoprecipitation (IP) lysates were also probed to demonstrate depletion of the target and the respective binding partner. H, I, N-cadherin and β-catenin coimmunoprecipitate in SC–DRG cocultures. The immunoprecipitation using either the N-cadherin (H) or β-catenin (I) antibody clearly demonstrates a robust association, which is enhanced after the induction of myelination by ascorbic acid. The asterisks highlight the limited association between N-cadherin and β-catenin before the induction of myelination. The IgG heavy chain illustrates the proper loading of the samples.
