Figure 1.
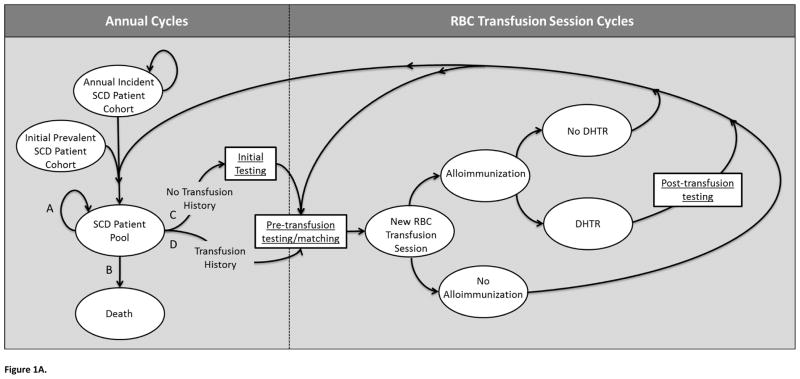
Figure 1A. Illustration of Markov model.
A portion of the simulation operates through annual cycles, where simulated patients enter the model as part of an “Annual Incident SCD Patient Cohort,” or as part of the “Initial Prevalent SCD Patient Cohort.” Each annual incident cohort joins this initial prevalent cohort to form the “SCD Patient Pool.” Individuals of this pool may follow one of four paths each year (A, B, C, or D). Path A is defined by continuing in the SCD Patient Pool without undergoing transfusion therapy. Path B reflects leaving the simulation via death. Path C and D allow simulated patients to undergo chronic transfusion therapy. Patients with no previous transfusion history follow Path C, which first leads to an “Initial Testing” phase, and then to “Pre-transfusion testing/matching,” while patients with a previous history of transfusion progress directly to “Pre-transfusion testing/matching” (Path D). Patients undergoing chronic transfusion therapy (Paths C or D) enter a portion of the model with each cycle defining a single RBC transfusion session. While patients following paths A or B continue through a series of annual cycles, others transition to “New RBC Transfusion Session,” Each transfusion session could lead to an alloimmunization event, which could result in a DSTR or DHTR, or only a positive antibody screen. In the event that a transfusion session resulted in a DHTR, “Post-transfusion testing” would be conducted. Regardless, patients would subsequently either return to “Pre-transfusion testing/matching” for another transfusion session within the same annual cycle or transition back to the “SCD Patient Pool” if no further transfusion sessions were required during the year.
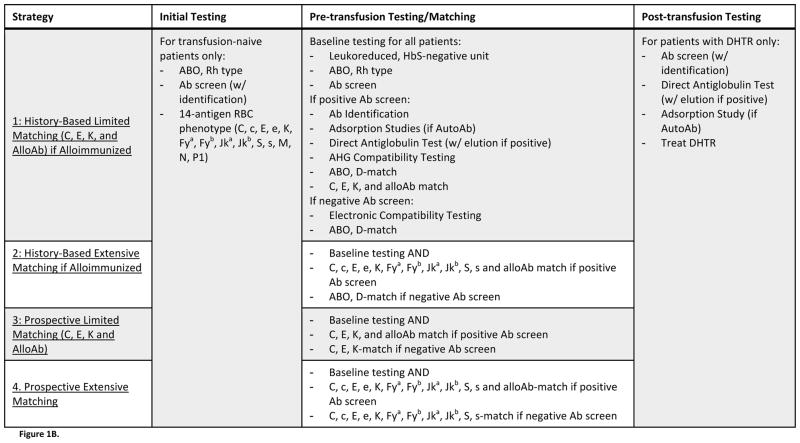
Figure 1B. Description of Evaluated Strategies. Four strategies of antigen-matching, which differed by the protocols used for preventing and managing alloimmunization, were evaluated. All strategies incorporated identical “Initial Testing” and “Post-transfusion Testing” procedures, but strategies differed by the procedure implemented for “Pre-transfusion Testing/Matching.” Strategies 1 and 2 characterized history-based antigen-matching, where only those patients who had previously formed alloantibodies would receive antigen-matched blood. Strategies 3 and 4 characterized prospective antigen-matching, where all patients – regardless of alloimmunization history – would receive antigen-matched blood. Under Strategies 1 and 3, antigen-matching would be limited, with units screened for C, E, K, and any other antigens against which the patient had formed alloantibodies. Under Strategies 2 and 4, however, antigen-matching would be extensive, with units screened for 11 antigens (C, c, E, e, K, Fya, Fyb, Jka, Jkb, S, s). Under any matching protocol, compatible units would be negative for any of the screened antigens that the patient lacked, and for any other antigens against which patients had formed alloantibodies.