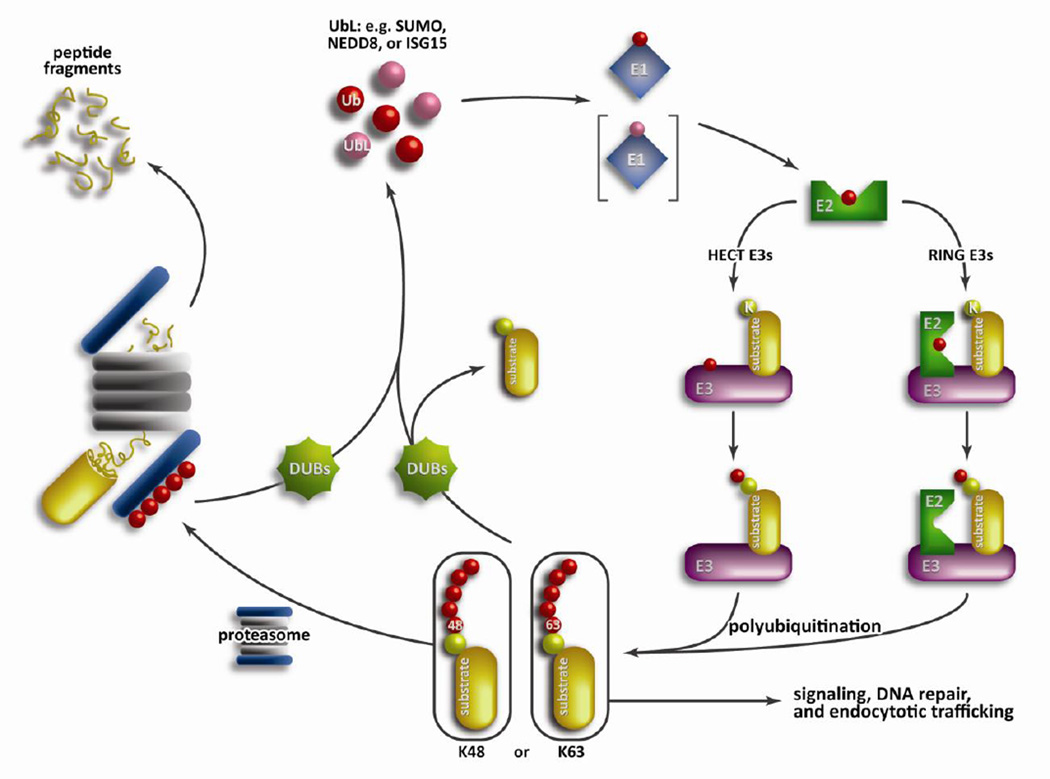
Figure 1. Overview of the UPS.
Posttranslational protein modification by ubiquitin (or ubiquitin-like proteins, UbLs) requires a cascade of three increasingly diverse enzymes: an E1 ubiquitin activating enzyme, an E2 ubiquitin conjugating enzyme, and an E3 ubiquitin ligase. Protein ubiquitination starts with an E1 forming a high energy thioester bond with free ubiquitin, which is recognized and transferred to the E2 enzyme. Next, an E3 ubiquitin ligase forms a complex with the E2 enzyme to mediate the transfer of ubiquitin in either a direct (RING family) or indirect (HECT family) manner. Polyubiquitin chains are formed through linkages of one of the seven lysines present on ubiquitin with K48-linked chains being targeted towards the proteasome. The 19S cap of the 26S proteasome recognizes the polyubiquitin chain and then unfolds and degrades the protein into small peptide fragments in the 20S core particle.