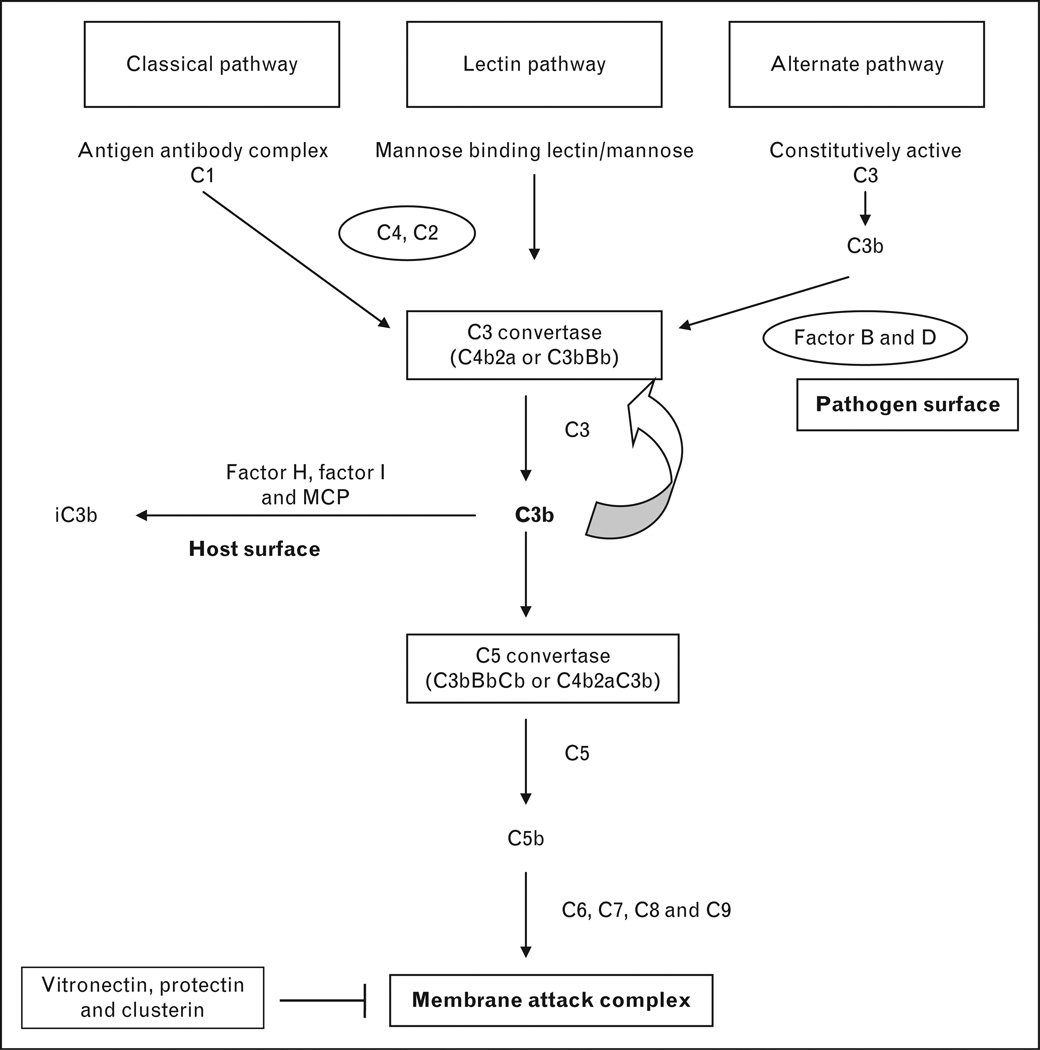
FIGURE 1.
Normal complement activation cascade and its regulation. This figure illustrates the normal complement pathways and the factors that protect the host from injury. Complement can be activated by three different pathways: classical, lectin and alternate pathways. C3 convertase from the classical and lectin pathways is generated by antigen antibody complex and interaction of mannose residues on the microbial surface with mannose binding lectin, respectively, in the presence of C4 and C2. The alternate pathway is constitutively active at low levels generating C3b. C3b binds with complement factor B, which is cleaved by complement factor D to generate C3bBb; the alternate pathway C3 convertase. Thus, all three complement pathways converge at C3 convertase. Either of the C3 convertases can bind to C3b to generate C5 convertase (C3bBbC3b or C4b2aC3b). Either of the C5 convertases can cleave C5 to C5b, which then binds to C6, C7, C8 and C9 to generate membrane attack complex. The regulation of complement activation is critical to the host. The important complement regulatory proteins associated with aHUS are complement factor H, complement factor I and MCP. In the presence of complement factor H and MCP, complement factor I cleaves C3b to inactive C3b (iC3b). The carbohydrate content of the cell membrane determines whether complement factor B or H binds to C3b. Eukaryotes have polyanions on their cell surface and C3b in that instance will bind preferentially to complement factor H and C3b is inactivated. Prokaryotes (bacteria) lack polyanions and complement factor B binds C3b to generate C3 convertase and further activation of the complement system. The formation of membrane attack complex is inhibited by vitronectin, clusterin and protectin. MCP, membrane cofactor protein.