Abstract
Objective
Atherosclerosis is an inflammatory disease with multiple underlying metabolic and physical risk factors. Bone morphogenic protein 4 (BMP4) expression is increased in endothelium in atherosclerosis-prone regions and is known to induce endothelial inflammation, endothelial dysfunction and hypertension. BMP actions are mediated by two different types of BMP receptors (BMPRI and II). Here we show a surprising finding that loss of BMPRII expression causes endothelial inflammation and atherosclerosis.
Approach and Results
Using BMPRII siRNA and BMPRII+/− mice, we found that specific knockdown of BMPRII, but not other BMP receptors (Alk1,Alk2, Alk3, Alk6, ActRIIa and ActRIIb) induced endothelial inflammation in a ligand-independent manner by mechanisms mediated by reactive oxygen species (ROS), NFκB, and NADPH oxidases. Further, BMPRII+/−ApoE−/− mice developed accelerated atherosclerosis compared to BMPRII+/+ApoE−/− mice. Interestingly, we found that multiple pro-atherogenic stimuli such as hypercholesterolemia, disturbed flow (d-flow), pro-hypertensive angiotensin II (AngII), and the pro-inflammatory cytokine, tumor necrosis factor-alpha (TNFα), downregulated BMPRII expression in endothelium, while anti-atherogenic stimuli such as stable flow (s-flow) and statin treatment upregulated its expression in vivo and in vitro. Moreover, BMPRII expression was significantly diminished in human coronary advanced atherosclerotic lesions. Also, we were able to rescue the endothelial inflammation induced by BMPRII knockdown by overexpressing the BMPRII wild-type, but not by the BMPRII short-form lacking the carboxyl-terminal tail region.
Conclusions
These results suggest that BMPRII is a critical, anti-inflammatory and anti-atherogenic protein that is commonly targeted by multiple pro- and anti-atherogenic factors. BMPRII may be used as a novel diagnostic and therapeutic target in atherosclerosis.
Keywords: atherosclerosis, endothelial cell, blood flow, BMPRII, signal transduction
Atherosclerosis is an inflammatory disease1, 2, associated with multiple systemic risk factors, including hypercholesterolemia, hypertension, diabetes, smoking and physical inactivity3-5. Despite the presence of these systemic risk factors, atherosclerosis preferentially occurs in specific focal regions of branched or curved arteries exposed to disturbed flow (d-flow), suggesting a potential interaction between hemodynamic conditions and systemic risk factors in atherogenesis. More specifically, disturbed flow induces endothelial inflammation, which may prime these arterial regions for atherogenesis in the presence of the aforementioned systemic risk factors1-5. Indeed, we have recently demonstrated that exposure of pristine, straight common carotid artery to disturbed flow by partial carotid ligation leads to rapid and robust atherosclerosis development in ApoE−/− mice fed a high-fat diet6, 7. However, the underlying mechanisms by which these synergistic effects lead to initiation and progression of atherosclerosis are still unclear.
Previously, we showed that bone morphogenic protein 4 (BMP4) is induced by disturbed flow in endothelial cells causing endothelial inflammation and dysfunction in an NFκB and NADPH oxidase-dependent manner8-10. In addition, BMP2 and BMP4 expression is increased in human atherosclerotic plaques and human coronary artery endothelium overlying early atherosclerotic lesions10, 11. Furthermore, we showed that chronic infusion of BMP4 causes systemic hypertension by stimulating activation of arterial NADPH oxidases and endothelial dysfunction subsequently in mice12. Moreover, overexpression of a BMP antagonist, matrix gla protein or treatment with a pharmacological BMP inhibitor protect against atherosclerosis13, 14. These results clearly suggest the role of BMPs in vascular disease, but specific BMP receptors that mediate this effect is unknown.
BMP actions are mediated by two different types of BMP receptors (BMPR); Type I receptors (Alk1, Alk2, Alk3, and Alk6) and Type II receptors (BMPRII, ActRIIa and ActRIIb)15, 16. Upon binding to BMPs, Type I BMPRs are activated by BMPRII and subsequently activate the canonical SMAD-1/5/8-dependent and –independent signaling pathways17, 18. Interestingly, it has been shown that loss-of-function mutations of BMPRII are linked to primary pulmonary hypertension in humans19, demonstrating its importance in pulmonary artery biology and pathophysiology. However, it is unknown whether the genetic mutations or changes in BMPRII expression have any association with conduit arterial diseases such as atherosclerosis.
We initially hypothesized that BMP4-induced endothelial inflammation would be mediated by BMPRII and that its knockdown would prevent the BMP4 effect. However, we found a surprising, paradoxical result that loss of BMPRII in endothelial cells caused robust inflammation and atherosclerosis. Further studies showed that the pro-inflammatory effect of BMPRII knockdown was unique to this receptor member as knockdown of other BMP receptors did not induce endothelial inflammation. Moreover, we found that multiple pro-atherogenic factors, either individually or in combination, resulted in a common response, loss of BMPRII in endothelial cells in vitro and in vivo. We also found loss of BMPRII expression in human coronary arteries with advanced atherosclerotic lesions.
METHODS
For detailed Materials and Methods please see online Data Supplement.
RESULTS
Loss of BMPRII induces endothelial inflammation in vitro and in vivo
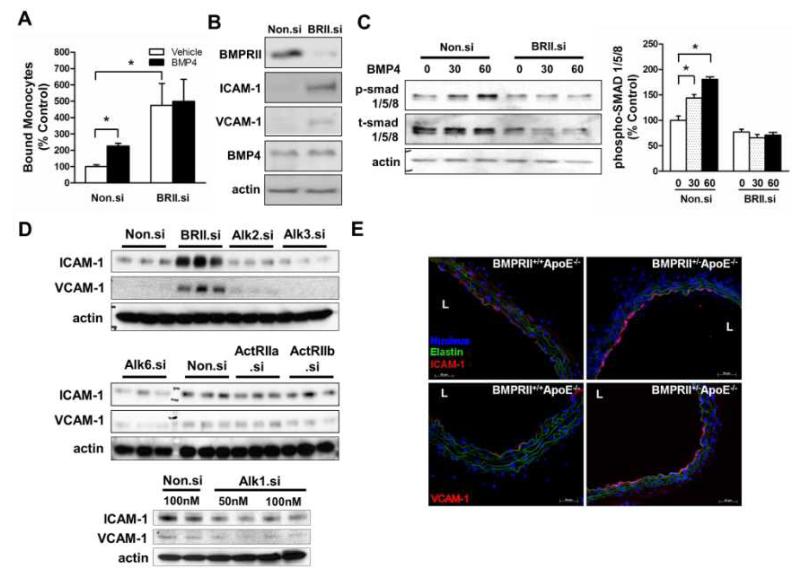
To examine whether BMPRII mediates BMP4-dependent endothelial inflammation, endothelial cells were treated with either BMPRII siRNA (BRII.si) or control siRNA (Non.si) in the presence or absence of BMP4, and monocyte adhesion assays were performed. As expected, BMP4 increased monocyte adhesion by 2-fold over siRNA control in HUVECs (Fig. 1A). To our surprise, however, knockdown of BMPRII markedly elevated basal monocyte adhesion by almost 5-fold over controls, independent of BMP4 (Fig. 1A). To determine the underlying mechanisms for this unexpected result, expression of cell adhesion molecules (ICAM-1 and VCAM-1), BMP4, and BMPRII was examined in HUVECs. BMPRII siRNA reduced BMPRII expression by ~90%, while ICAM-1 and VCAM-1 expression was increased by 6- and 3-fold, respectively, compared to non-silencing control, but basal BMP4 expression was unaffected (Fig. 1B and Suppl. Fig. 1A). We next investigated the effect of BMPRII knockdown on activation of the canonical BMP pathway by examining SMAD1/5/8 phosphorylation20-22 in HUVECs. As expected, BMP4 treatment induced SMAD1/5/8 phosphorylation in a time-dependent manner, which was blocked by BMPRII siRNA (Fig. 1C), consistent with a previous report23. This suggests that BMPRII siRNA effectively inhibits the canonical signaling pathway in response to BMP4, while paradoxically inducing endothelial inflammation.
Figure 1. Loss of BMPRII induces endothelial inflammation in vitro and in vivo.
(A, B, C) HUVECs were transfected with BMPRII siRNA (BRII.si) or non-silencing control siRNA (Non.si). In (A), cells were then treated with BMP4 (100 ng/ml, 4 h), and THP1 monocyte adhesion to ECs was determined (mean ± SEM, n=6, *p < 0.05). In (B), cells were lysed and analyzed by Western blot with antibodies to BMPRII, ICAM-1 and VCAM-1, using β-actin as a loading control. In (C), cells were treated with BMP4 and cell lysates were analyzed with phospho-smad1/5/8 or total smad1/5/8 antibodies, and the Western band intensities were quantified (*p<0.05, n=3). (D) HAECs transfected with siRNAs specific for BMPRII, Alk1, Alk2, Alk3, Alk6, ActRIIa, ActRIIb or control (Non.si) were analyzed by Western blots with BMPRII, VCAM-1 and ICAM-1 antibodies. (E) Representative images show immunostaining of frozen sections of thoracic aortas obtained from BMPRII+/−ApoE−/− and BMPRII+/+ApoE−/− mice (n=6 each) using ICAM-1 and VCAM-1 antibodies (red). Nuclei stained with DAPI (blue) and auto fluorescence signals showing elastic laminas are shown in green. L: lumen.
We next tested whether this pro-inflammatory effect is specific to BMPRII knockdown. To compare the specificity of each BMP receptor, endothelial cells were treated with siRNAs specific for other BMP receptors (Type I: Alk1, Alk2, Alk3 and Alk6 and Type II: ActRIIa, ActRIIb, or BMPRII). Only BMPRII siRNA was able to induce ICAM-1 and VCAM-1 expression markedly above control siRNA levels, while all other BMP receptor siRNAs tested did not (Fig. 1D). Interestingly, Alk1 knockdown decreased endothelial inflammation, which suggests that basal expression of ICAM-1 and VCAM-1 is mediated by ligands such as BMPs or TGFβ existing in the culture media. Also, Alk3 and Alk6, like Alk1 showed a moderate downregulation of ICAM-1 compared to the Non.si controls indicating that these receptors might mediate the proinflammatory effects attributable to these ligands which may be present in the culture media or produced by the cells themselves. These findings suggest that specific loss of BMPRII, but not other BMP receptors, triggers an endothelial inflammatory response (Fig. 1D and Suppl. Fig. 1D-F).
To validate whether this unexpected in vitro finding was also relevant in vivo, we examined expression of ICAM-1 and VCAM-1 in arterial walls of BMPRII-deficient ApoE knockout mice (BMPRII+/−ApoE−/−) in comparison to BMPRII+/+ApoE−/− littermate control mice. Since homozygotic knockout of BMPRII causes embryonic lethal phenotype24, heterozygotic BMPRII+/− mice were used. To rule out the effect of high-fat diet on our experimental model, the mice used in the study were fed a standard chow diet. Expression of both ICAM-1 and VCAM-1 was significantly increased in the arterial endothelium of BMPRII+/−ApoE−/− compared to BMPRII+/+ApoE−/− mice (Fig. 1E; Suppl. Fig. 1H). These results suggest that specific BMPRII knockdown induces endothelial inflammation in a BMP4-independent manner both in vitro and in vivo.
Loss of BMPRII induces endothelial inflammation by mechanisms mediated by ROS, NADPH oxidase, and NFκB activity
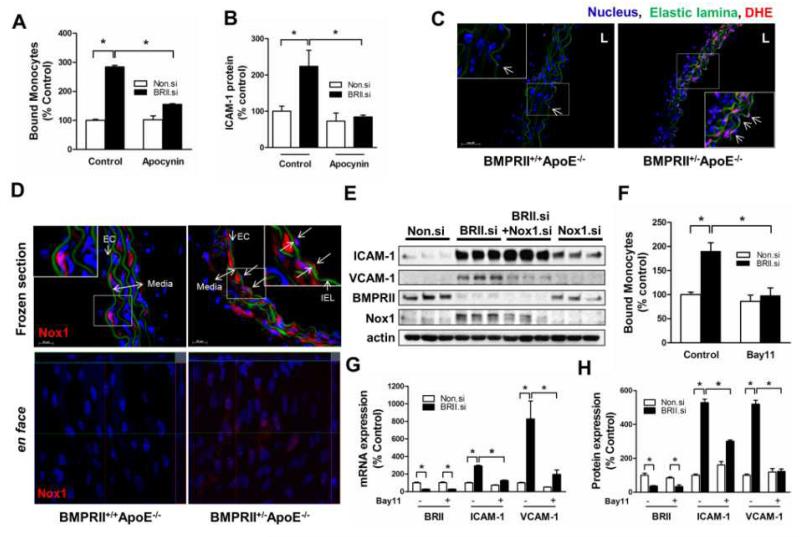
Next we studied the mechanisms by which BMPRII knockdown induces endothelial inflammation. Since reactive oxygen species (ROS) play an important role in endothelial inflammation and atherosclerosis6, 9, we tested whether the pro-inflammatory effect of BMPRII was mediated by ROS. Knockdown of BMPRII increased ROS production in HUVECs as measured by Amplex-Red. This increased ROS production was significantly blunted by the cell-permeable ROS scavenger polyethylene glycol (PEG)-catalase (Suppl. Fig. 2A), implicating a potential role for ROS in BMPRII-mediated endothelial inflammation.
Since Nox1 is a NADPH oxidase member producing ROS involved in endothelial inflammation9, we tested the effect of apocynin (a NADPH oxidase inhibitor and ROS scavenger)25 on BMPRII knockdown-induced inflammation in HUVECs. Treatment with apocynin significantly inhibited increased monocyte adhesion and ICAM-1 expression following BMPRII siRNA (Fig. 2A-B, Suppl. Fig. 2B-C). To validate these in vitro findings, we measured in vivo ROS production in the aortic walls of BMPRII+/−ApoE−/− and BMPRII+/+ApoE−/− mice by DHE staining9. Increased ROS production was observed in the aortic wall, including endothelium (arrows in the inset) and smooth muscle cells of BMPRII+/−ApoE−/− compared to BMPRII+/+ApoE−/− mice (Fig. 2C). ROS production in the aortic endothelium coincided with Nox1 expression in BMPRII+/−ApoE−/−, which was not detected in BMPRII+/+ApoE−/− mice (Fig. 2D), indicating a potential link between BMPRII reduction, Nox1 induction, and ROS production in the arterial wall. This was further demonstrated in HUVECs, which showed that BMPRII knockdown induced Nox1 expression (Suppl. Fig. 2D; Fig. 2E). Moreover, simultaneous knockdown of Nox1 and BMPRII significantly prevented VCAM-1 expression, but not ICAM-1, (Fig. 2E; Suppl. Fig. 2E), showing a specific effect of the BMPRII knockdown on VCAM-1 by a Nox1-dependent mechanism.
Figure 2. Loss of BMPRII induces endothelial inflammation by mechanisms dependent on ROS, NADPH oxidase, and NFκB activity.
(A, B) HUVECs transfected with BMPRII siRNA or Non.si were also treated with apocynin (60 μM, 2 days) or vehicle, and monocyte adhesion assay (A) or Western blot analysis (B) of cell lysates using ICAM-1 antibody were performed (mean ± SEM, n=4, *p<0.05). (C, D) Thoracic aorta sections of BMPRII+/+ApoE−/− and BMPRII+/− ApoE−/− mice were stained with dihydroethidium for ROS detection (C) or Nox1 antibody (D). Shown are representative confocal microscopy images (n=6 each). The insets show magnified views of endothelial regions. Nuclei (blue) and elastic laminas (green) are shown. L: lumen. Also shown are en face confocal images of Nox1 antibody staining (lower panels, D). (E) HUVECs were transfected with Non.si, BMPRII siRNA, BMPRII siRNA+Nox1 siRNA, or Nox1 siRNA for 2 days and analyzed by Western blots using ICAM-1, VCAM-1, BMPRII, Nox1 and β-actin antibodies. (F-H) HUVECs transfected with BMPRII siRNA or Non.si were treated with Bay11-7082 (10μM, 24 h) before monocyte adhesion assay (F), qPCR analysis for BMPRII, ICAM-1 and VCAM-1 (G), and Western blots with BMPRII, ICAM-1 and VCAM-1 antibodies (H), (n=4, *p<0.05).
Since induction of ICAM-1 and VCAM-1 is known to be regulated by NFκB, we tested the role of NFκB signaling pathway in BMPRII-knockdown-induced endothelial inflammation. Treatment of HUVECs with the NFκB inhibitor BAY 11-7082 significantly inhibited monocyte adhesion, as well as ICAM-1 and VCAM-1 mRNA and protein expression induced by BMPRII knockdown (Fig. 2F-H, Suppl. Fig. 2F). Collectively, these findings suggest that knockdown of BMPRII in endothelial cells stimulates NADPH oxidase and ROS production, which in turn mediates endothelial inflammatory responses including ICAM-1 and VCAM-1 expression, and monocyte adhesion by a NFκB-dependent pathway.
BMPRII deficiency exacerbates atherosclerosis development in ApoE−/− mice
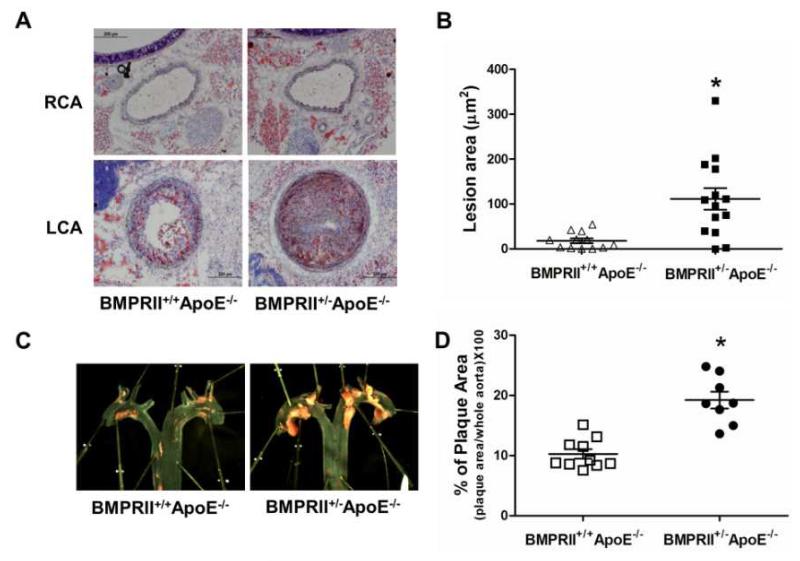
We then tested the hypothesis that BMPRII-deficiency exacerbates atherosclerosis using acute and chronic mouse models. We compared atherosclerosis development in the carotid arteries of ApoE−/− mice using an acute partial ligation model of atherosclerosis as we recently described6, 7. As expected, partial ligation of left carotid artery (LCA) rapidly induced atherosclerosis in BMPRII+/+ApoE−/− by two weeks post-ligation on a high-fat diet, while the contralateral right carotid artery (RCA) remained lesion-free (Fig. 3A). In comparison, BMPRII-deficiency in BMPRII+/−ApoE−/− mice increased LCA lesion area by more than 5-fold compared to BMPRII+/+ApoE−/− littermate controls (Fig. 3A-B). Increased atherosclerosis in the BMPRII-deficient mice showed correlation with CD45+ leukocyte infiltration (Suppl. Fig. 3), but was not due to exacerbated hypercholesterolemia, or abnormal serum lipid profiles compared to BMPRII+/+ApoE−/− littermate controls (Supplementary Table 1). Next, chronic atherosclerosis development was examined in the aortic arches of BMPRII+/−ApoE−/− and BMPRII+/+ApoE−/− mice fed a high-fat diet. Treatment with high-fat diet for two months induced atherosclerosis in the aortic arch of BMPRII+/+ApoE−/−, as expected. In comparison, BMPRII-deficiency increased plaque burden by two-fold in BMPRII+/−ApoE−/− mice (Fig. 3C-D). These results obtained from the two different models of atherosclerosis strongly indicate that genetic haplodeficiency of BMPRII is sufficient to exacerbate atherosclerosis development.
Figure 3. BMPRII deficiency exacerbates atherosclerosis development in ApoE−/− mice.
(A) BMPRII+/−ApoE−/− (n=12) and littermate control BMPRII+/+ApoE−/− mice (n=14) were partially ligated and fed a high-fat diet for 2 weeks. Frozen sections from the ligated LCA (d-flow) and contralateral RCA (s-flow) were stained with Oil-Red-O as shown by representative microscopy images. (B) Atherosclerotic lesion area was quantified (*p<0.001). (C) BMPRII+/−ApoE−/− (n=8) and BMPRII+/+ApoE−/− mice (n=10) were fed the high-fat diet for 2 months. Aortic arch regions were stained with Oil-Red-O as shown, and (D) atherosclerotic lesion areas determined (*p<0.05).
BMPRII expression is down-regulated by pro-atherogenic risk factors, while up-regulated by anti-atherogenic conditions
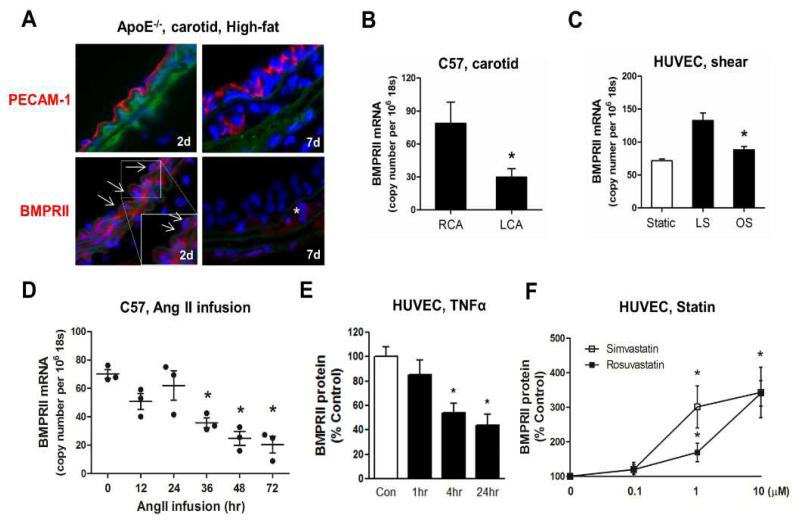
We next asked whether BMPRII expression in arterial endothelium is regulated by conditions or stimuli relevant to pathophysiology of human atherosclerosis. Since atherosclerosis is caused by multiple risk factors, we hypothesized that various pro-atherogenic conditions, such as hypercholesterolemia, d-flow, hypertension and pro-inflammatory cytokines, either individually or in combination, downregulate BMPRII expression, which in turn drives endothelial inflammation and atherosclerosis. We first tested whether BMPRII expression could be decreased by d-flow and hypercholesterolemia using our carotid partial ligation model of d-flow-induced atherosclerosis in ApoE−/− mice fed a high-fat diet (Fig. 4A)6, 26. Although we did not notice any remarkable change in the BMPRII expression at an early time point (2 day post-ligation), BMPRII expression became undetectable in flow-disturbed LCA endothelium and was markedly reduced in smooth muscle cells at 1 week post-ligation. In contrast, BMPRII expression in the contralateral RCA remained relatively unchanged at the 1 week time point (Suppl. Fig. 4). These findings suggest that d-flow and hypercholesterolemia in combination downregulated BMPRII expression, while 1 week of hypercholesterolemia alone was not sufficient to decrease BMPRII level. Interestingly, we did not observe any BMPRII staining in the intima at 1 week post-ligation (Suppl. Fig. 4), suggesting that the leukocytes infiltrating the intima at this time point as we recently demonstrated26 do not express detectable level of BMPRII expression.
Figure 4. BMPRII expression is reduced by pro-atherogenic risk factors, while upregulated by anti-atherogenic conditions.
(A) ApoE−/− mice were partially ligated and fed the high-fat diet for 2 or 7 days. Frozen sections of ligated LCA exposed to d-flow were stained with PECAM-1 or BMPRII antibody (red) and representative confocal microscopy images are shown (n=6). DAPI staining of nuclei (blue) and elastic laminas (green) are shown. Arrows and * point to endothelial cells and intima leukocytes, respectively. (B) Endothelial-enriched RNAs were isolated from RCA and LCA of C57Bl6 mice at 48 h post-partial ligation, and BMPRII mRNA was determined by qPCR (n=4, *p<0.05). (C) HUVECs were exposed to static control, laminar shear (LS) or oscillatory shear (OS) in vitro for 24 h, and BMPRII expression was determined by qPCR (n=4, *p<0.05). (D) Endothelial-enriched RNAs were isolated from thoracic aortas of C57Bl6 mice, following AngII infusion for 0 to 72 h, and BMPRII was determined by qPCR (n=4, *p<0.05). (E, F) HUVECs were treated with TNFα (E) or simvastatin or rosuvastatin for 24 h (F).
To determine whether flow itself could downregulate BMPRII expression in the arterial endothelium, endothelial-enriched RNA was collected from the LCA and RCA of C57Bl6 mice fed a chow diet at 48 h post ligation as we previously described6, 7. BMPRII mRNA expression was decreased by more than 2-fold in the LCA endothelium compared to RCA (Fig. 4B). This was further demonstrated in HUVECs in vitro, which we exposed to unidirectional, laminar (LS) and oscillatory shear stress (OS) using a cone-and-plate shear device to mimic s-flow and d-flow in vivo, as we described previously8, 27. BMPRII expression was increased in HUVECs exposed to LS compared to OS and static control (Fig. 4C). These findings further substantiate that pro-atherogenic d-flow downregulates while anti-atherogenic s-flow upregulates BMPRII expression in endothelial cells both in vivo and in vitro.
Next we tested whether angiotensin II (AngII), a key regulator of hypertension, and pro-inflammatory and pro-atherogenic agent, downregulates BMPRII expression in aortic endothelium. AngII infusion in C57BL/6 mice decreased BMPRII mRNA expression in a time-dependent manner, measured using endothelial-enriched RNA isolated from thoracic aortas at 36 to 72 h post-infusion (Fig. 4D). Lastly, we examined the role of pro-inflammatory cytokines using TNFα as a well-known prototypical cytokine that induces acute endothelial cell inflammation26, 28, on BMPRII expression in HUVECs. TNFα significantly inhibited BMPRII expression in a concentration- and time-dependent manner (Fig. 4E, Suppl. Fig. 5A). Conversely, we tested whether BMPRII expression could be upregulated or maintained by anti-atherogenic stimuli. For this purpose, we chose statins as they are known to provide anti-inflammatory and anti-atherogenic benefits in addition to their well-known lipid-lowering effect29, 30. Recently, simvastatin has been shown to upregulate the expression of BMPRII in different cell types in vitro including lung microvascular endothelial cells31. Treatment of HUVECs with 3 different statins (simvastatin, rosuvastatin and mevastatin) significantly increased BMPRII expression both at the mRNA and protein levels (Fig. 4F and Suppl. Fig. 5B-D). These results show that anti-atherogenic conditions such as s-flow and statin treatment prevent the loss of BMPRII expression, while multiple pro-atherogenic conditions including hypercholesterolemia, d-flow, AngII and TNFα downregulate BMPRII expression.
Loss of BMPRII in human coronary arteries with advanced atherosclerotic plaques
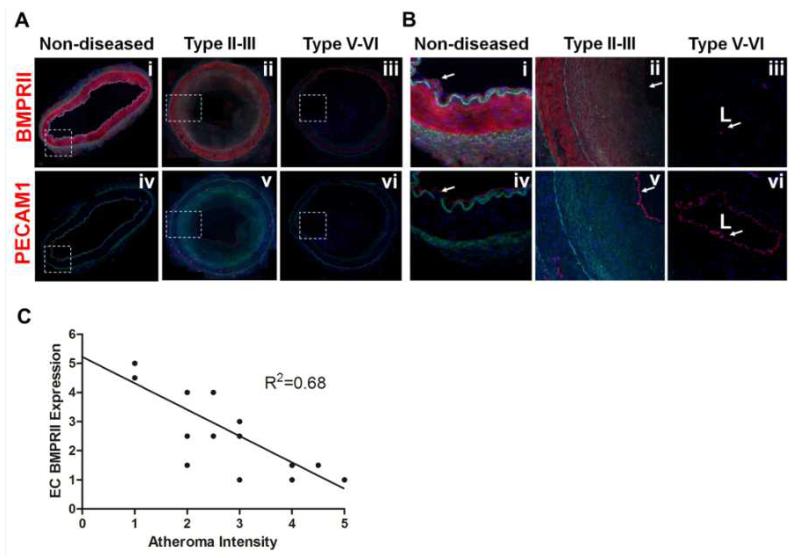
To examine whether our findings in in vitro settings and mouse models are relevant to human atherosclerosis, BMPRII expression was determined in human coronary arteries with atherosclerosis. Immunohistochemical staining showed that BMPRII expression was readily detected in non-diseased human coronary artery wall, including luminal endothelium and smooth muscle cells, but not in adventitia (Fig. 5A-B). However, BMPRII expression began to decrease (Fig. 5A-B; ii and v) in the coronary artery wall in early and intermediate lesions (Type II-III) and was nearly undetectable (Fig. 5A-B; iii and vi) in advanced lesions (Type V-VI)32, 33 BMPRII staining was also not detected in intimal regions known to contain leukocyte accumulations, consistent with the above finding in mouse (Fig. 4A). Overall, BMPRII expression in coronary artery endothelium showed a significant negative correlation (R2=0.68, n=14 patients) with atheroma intensity (Fig. 5C), suggesting that a similar relationship exists between BMPRII loss and atherosclerosis development in humans as demonstrated in our mouse models.
Figure 5. Loss of BMPRII in human coronary arteries with advanced atherosclerotic plaques.
(A, B) Representative confocal microscopy images of human coronary arteries containing various stages of atherosclerotic lesions were stained with antibodies to BMPRII (i-iii) and PECAM-1 (iv-vi), shown in red. DAPI (blue); auto fluorescence matrix signals (green). Images shown in (B) are magnified regions indicated by broken boxes in (A). (C) The graph shows semi-quantification of BMPRII staining intensity in the endothelial layer as a function of atheroma severity carried out by two blinded investigators (n=14).
Overexpression of BMPRII rescues endothelial inflammation caused by BMPRII knockdown
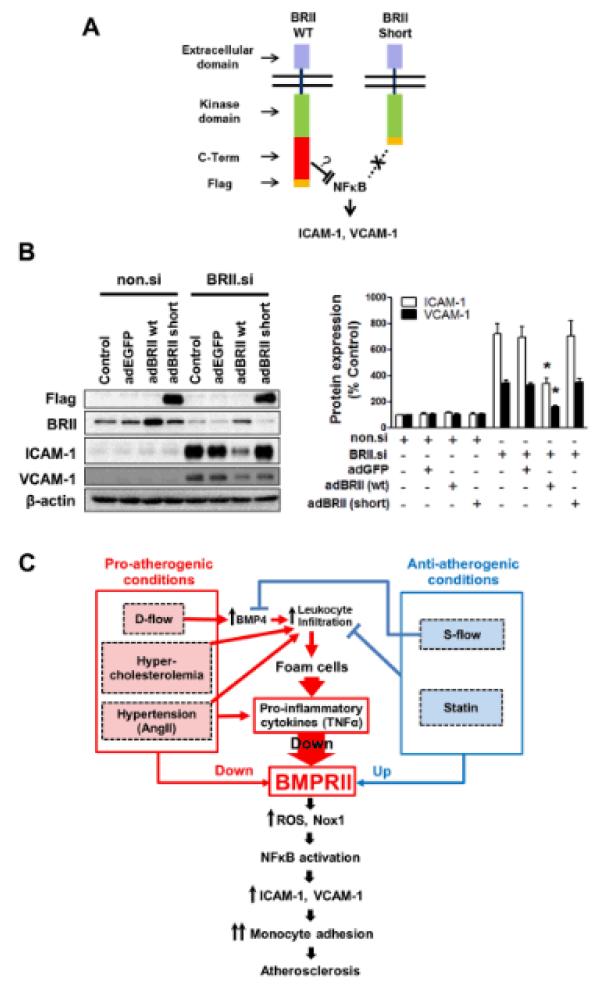
Next we tested whether we could reverse the BMPRII knockdown-induced endothelial inflammation by overexpressing BMPRII. To this end, we overexpressed BMPRII wild-type or short-form, lacking the C-terminal tail domain using adenoviral constructs in endothelial cells treated with BMPRII siRNA. Overexpression of BMPRII wild-type, but not the short-form, was able to blunt the endothelial expression of ICAM-1 and VCAM-1 induced by BMPRII knockdown (Fig. 6A and B). This result suggests that the C-terminal tail region of BMPRII plays a critical role in regulating ICAM-1 and VCAM-1 expression in response to BMPRII knockdown.
Figure 6. Endothelial inflammation induced by BMPRII knockdown can be rescued by overexpressing BMPRII WT, but not by the BMPRII short form.
(A) shows a schematic of BMPRII WT and BMPRII Short-form, indicating the C-terminal FLAG tag in both constructs. (B) HUVECs treated with non.si and BRII.si were further infected with Adenoviral BMPRII WT or BMPRII Short for 24 h. Representative blots show expression of ICAM-1 and VCAM-1 with FLAG tagged BMPRII Short form and BMPRII WT expression and β-actin as controls. The bar graph shows the quantification of ICAM-1 and VCAM-1 expression of Western blots (n=3; *p<0.05). (C) The schematic shows summary and working hypothesis that pro-atherogenic conditions downregulate BMPRII expression, which in turn induces endothelial inflammation and atherosclerosis. Anti-atherogenic conditions upregulate endothelial BMPRII expression.
DISCUSSION
Our serendipitous finding that the loss of BMPRII induces endothelial inflammation and atherosclerosis came as a surprise at first since we initially hypothesized that knockdown of the receptor mediating BMP4 action would prevent endothelial inflammation. To the contrary, we found that knockdown of BMPRII in endothelial cells induced monocyte adhesion through the expression of ICAM-1 and VCAM-1 (Fig. 1). This increase in the adhesion molecule expression and monocyte adhesion was independent of the BMP4 treatment indicating that BMPRII plays a critical role in endothelial inflammation, perhaps in a BMP ligand-independent manner. Further in vivo studies showed that loss of BMPRII induced endothelial inflammation (Fig. 1 and 2) and atherosclerosis in BMPRII+/− ApoE−/− mice (Fig. 3). Our data also showed that BMPRII expression was progressively lost as advanced atherosclerotic plaques formed in human coronary arteries (Fig. 5). The progressive loss of BMPRII was also found in our mouse model of atherosclerosis (Fig. 4A). Together, these results suggest that loss of BMPRII may be a common event occurring both in human atherosclerosis and mouse models of atherosclerosis.
While our data on loss of BMPRII expression in human coronary arteries with advanced lesions need to be interpreted with caution due to the small number of tissue samples (n=14 patients) used in the study, it does raise the possibility that loss of BMPRII in atherosclerosis may be an important pathophysiological molecular event that has not been appreciated thus far. Although genomic mutations or downregulation of BMPRII have been linked to human primary pulmonary hypertension19, human secondary pulmonary hypertension34, and rat hypoxic pulmonary hypertension35, they have never been implicated in atherosclerosis. Primary pulmonary hypertension predominantly occurs in young women with a median age at diagnosis of 36.4 years36-39. The rarity of the disease19, 37, 38 and short survival duration [mean survival of 2.8 years40-43] of these patients may explain why BMPRII has not been previously implicated in more common vascular diseases such as atherosclerosis.
Our data indicate that seemingly disparate, multiple pro-atherogenic factors downregulate BMPRII expression through a potentially common pathway. For example, hypercholesterolemia, d-flow, the pro-hypertensive AngII, and the pro-inflammatory cytokine TNFα, all downregulated BMPRII expression in endothelial cells in vitro and in vivo (Fig. 4). One possibility is that these multiple pro-atherogenic risk factors converge on increased production of pro-inflammatory cytokines such as TNFα, acting as common effectors44. These pro-inflammatory cytokines produced by vascular wall cells and infiltrating leukocytes under various pro-atherogenic conditions may downregulate BMPRII expression. In contrast, anti-inflammatory conditions such as treatment with statins31 or s-flow increase or maintain BMPRII expression thereby preventing endothelial inflammation and atherosclerosis. BMPRII thus may serve as a common target of these pro- and anti-atherogenic risk factors (Fig. 6C).
We found that loss of BMPRII induced endothelial inflammation and atherosclerosis by the mechanisms mediated by NADPH oxidases, ROS production, and NFκB activation. How the loss of BMPRII triggers these signaling pathways needs to be further defined, but it may depend upon cell types and biological context23. Interestingly, we were able to rescue endothelial inflammation caused by BMPRII knockdown by overexpressing BMPRII wild-type, but not by the BMPRII short form. This suggests that the C-terminal tail domain of BMPRII plays a constitutive anti-inflammatory role and that its loss or reduction induces pro-inflammatory response. One potential mechanistic explanation for this is the unique “molecular docking-nature” of the long cytoplasmic tail domain of BMPRII, which is not found in other members of TGFβ/BMP receptor superfamily45. The long cytoplasmic tail domain of BMPRII is known to bind various signaling proteins including NFκB-p50 protein and cSrc46. Loss of the BMPRII, especially the C-terminal domain, may induce abnormal activation of these pathways, leading to NFκB activation and endothelial inflammation. Consistent with this idea, the pro-inflammatory effect of BMPRII knockdown was unique only to this receptor as knockdown of all other Type I and Type II BMP receptors examined in our study did not induce endothelial inflammation.
Other possibilities include that the loss of BMPRII may result in altered activation of non-BMP2/4 ligands such as BMP6/7 and BMP9/10 families as well as TGFβ, while inhibiting BMP2/4 pathway. In pulmonary artery smooth muscle cells, BMPRII deletion was shown to inhibit BMP2/4 signaling while augmenting BMP6/7 pathway via activation of ActRIIa receptor23. However, knockdown of ActRIIa, which is the primary receptor for BMP6/747, did not affect endothelial inflammation in our system, suggesting that endothelial inflammation caused by BMPRII knockdown is unlikely to be mediated by BMP6/7. BMP9 is known to be a vascular quiescence factor and binds to Alk1 and BMPRII along with a co-receptor48. However, our results showed that BMP9 and BMP10 neutralizing antibodies had no effect on endothelial inflammation caused by BMPRII knockdown, ruling out the potential role of these BMPs in our system (Suppl. Fig. 6A-C). Further, we showed that knockdown of Alk1, which could mediate TGFβ effect49, did not increase endothelial inflammation (Suppl. Fig. 1F). Together, these results suggest that endothelial inflammation induced by BMPRII reduction occurs in a ligand-independent manner.
We propose that multiple, pro-atherogenic risk factors such as hypercholesterolemia, hypertension, d-flow and inflammatory conditions, either independently or in combination, downregulate BMPRII expression through a common mechanism (Fig. 6C). Endothelial cells exposed to d-flow in athero-prone regions produce BMP4, which in turn induces an early endothelial inflammation resulting in intimal leukocytes infiltration. In the presence of additional risk factors such as hypercholesterolemic conditions, these leukocytes including macrophages and dendritic cells become foam cells and produce pro-inflammatory cytokines. These cytokines such as TNFα, and additional risk factors including hypertensive AngII and d-flow would then downregulate BMPRII, which triggers vicious cycle of inflammatory responses leading to atherosclerosis by the ROS and NFκB-dependent mechanisms (Fig. 6C).
In conclusion, we show that BMPRII is a common target of multiple pro-atherogenic factors and that its loss in arterial wall induces endothelial inflammation and atherosclerosis. BMPRII may be a critical, anti-inflammatory and anti-atherogenic protein and may be used as a novel diagnostic and therapeutic target in atherosclerosis.
Supplementary Material
SIGNIFICANCE.
Atherosclerosis is a multifactorial disease that usually develops many years before any clinical symptoms are manifest. The role of Bone morphogenic protein receptor (BMPRII) in atherosclerosis is not known. Here, using BMPRII siRNA and BMPRII+/− mice, we show a surprising finding that loss of BMPRII expression causes endothelial inflammation and atherosclerosis by mechanisms mediated by reactive oxygen species (ROS), NFκB, and NADPH oxidases. Interestingly, we found that multiple pro-atherogenic stimuli such as hypercholesterolemia, disturbed flow (d-flow), pro-hypertensive angiotensin II (AngII), and the pro-inflammatory cytokine, tumor necrosis factor-alpha (TNFα), downregulated BMPRII expression in endothelium, while anti-atherogenic stimuli such as stable flow (s-flow) and statin treatment upregulated its expression in vivo and in vitro. Endothelial inflammation induced by BMPRII knockdown was alleviated by overexpressing the BMPRII wild-type, but not by the BMPRII short-form lacking the carboxyl-terminal tail region. Therefore, BMPRII may be used as a novel diagnostic and therapeutic target in atherosclerosis.
Acknowledgments
We thank Dr. Akiko Hata for kindly providing the adenoviral constructs for BMPRII and Dr. Hideyuki Beppu for providing BMPRII+/− mice.
Sources of Funding
This work was supported by funding from NIH grants HL095070, HL70531, HHSN268201000043C (HJ and WRT), a World Class University Project (R31-2008-000-10010-0) from the Ministry of Science, Technology and Education of S. Korea (HJ) and Ada Lee Pete Correll Professorship.
Footnotes
Disclosures
None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 3.VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: Site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol. 2004;24:12–22. doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- 4.Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res. 1983;53:502–514. doi: 10.1161/01.res.53.4.502. [DOI] [PubMed] [Google Scholar]
- 5.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2011 update. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol. 2009;297:H1535–1543. doi: 10.1152/ajpheart.00510.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ni CW, Qiu H, Rezvan A, Kwon K, Nam D, Son DJ, Visvader JE, Jo H. Discovery of novel mechanosensitive genes in vivo using mouse carotid artery endothelium exposed to disturbed flow. Blood. 2010;116:e66–73. doi: 10.1182/blood-2010-04-278192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang K, Weiss D, Suo J, Vega JD, Giddens D, Taylor WR, Jo H. Bone morphogenic protein antagonists are coexpressed with bone morphogenic protein 4 in endothelial cells exposed to unstable flow in vitro in mouse aortas and in human coronary arteries: Role of bone morphogenic protein antagonists in inflammation and atherosclerosis. Circulation. 2007;116:1258–1266. doi: 10.1161/CIRCULATIONAHA.106.683227. [DOI] [PubMed] [Google Scholar]
- 9.Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, Boyd NL, Platt MO, Lassegue B, Griendling KK, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based nadph oxidase. Circ Res. 2004;95:773–779. doi: 10.1161/01.RES.0000145728.22878.45. [DOI] [PubMed] [Google Scholar]
- 10.Sorescu GP, Sykes M, Weiss D, Platt MO, Saha A, Hwang J, Boyd N, Boo YC, Vega JD, Taylor WR, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J Biol Chem. 2003;278:31128–31135. doi: 10.1074/jbc.M300703200. [DOI] [PubMed] [Google Scholar]
- 11.Dhore CR, Cleutjens JPM, Lutgens E, Cleutjens KBJM, Geusens PPM, Kitslaar PJEHM, Tordoir JHM, Spronk HMH, Vermeer C, Daemen MJAP. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001;21:1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- 12.Miriyala S, Gongora Nieto MC, Mingone C, Smith D, Dikalov S, Harrison DG, Jo H. Bone morphogenic protein-4 induces hypertension in mice: Role of noggin, vascular nadph oxidases, and impaired vasorelaxation. Circulation. 2006;113:2818–2825. doi: 10.1161/CIRCULATIONAHA.106.611822. [DOI] [PubMed] [Google Scholar]
- 13.Yao Y, Bennett BJ, Wang X, Rosenfeld ME, Giachelli C, Lusis AJ, Bostrom KI. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circ Res. 2010;107:485–494. doi: 10.1161/CIRCRESAHA.110.219071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derwall M, Malhotra R, Lai CS, Beppu Y, Aikawa E, Seehra JS, Zapol WM, Bloch KD, Yu PB. Inhibition of bone morphogenetic protein signaling reduces vascular calcification and atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:613–622. doi: 10.1161/ATVBAHA.111.242594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ten Dijke P, Korchynskyi O, Valdimarsdottir G, Goumans MJ. Controlling cell fate by bone morphogenetic protein receptors. Mol Cell Endocrinol. 2003;211:105–113. doi: 10.1016/j.mce.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 16.Miyazono K, Maeda S, Imamura T. Bmp receptor signaling: Transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine & growth factor reviews. 2005;16:251–263. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Hogan BL. Bone morphogenetic proteins in development. Curr Opin Genet Dev. 1996;6:432–438. doi: 10.1016/s0959-437x(96)80064-5. [DOI] [PubMed] [Google Scholar]
- 18.Massague J. How cells read tgf-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 19.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, 3rd, Loyd JE, Nichols WC, Trembath RC, The international pph consortium Heterozygous germline mutations in bmpr2, encoding a tgf-beta receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 20.Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. Journal of biochemistry. 2010;147:35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- 21.Lebrin F, Deckers M, Bertolino P, Ten Dijke P. Tgf-beta receptor function in the endothelium. Cardiovascular research. 2005;65:599–608. doi: 10.1016/j.cardiores.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 22.de Jesus Perez VA, Alastalo TP, Wu JC, Axelrod JD, Cooke JP, Amieva M, Rabinovitch M. Bone morphogenetic protein 2 induces pulmonary angiogenesis via wnt-beta-catenin and wnt-rhoa-rac1 pathways. J Cell Biol. 2009;184:83–99. doi: 10.1083/jcb.200806049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu PB, Beppu H, Kawai N, Li E, Bloch KD. Bone morphogenetic protein (bmp) type ii receptor deletion reveals bmp ligand-specific gain of signaling in pulmonary artery smooth muscle cells. J Biol Chem. 2005;280:24443–24450. doi: 10.1074/jbc.M502825200. [DOI] [PubMed] [Google Scholar]
- 24.Beppu H, Kawabata M, Hamamoto T, Chytil A, Minowa O, Noda T, Miyazono K. Bmp type ii receptor is required for gastrulation and early development of mouse embryos. Developmental Biology. 2000;221:249–258. doi: 10.1006/dbio.2000.9670. [DOI] [PubMed] [Google Scholar]
- 25.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular nadph oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 26.Alberts-Grill N, Rezvan A, Son DJ, Qiu H, Kim CW, Kemp ML, Weyand CM, Jo H. Dynamic immune cell accumulation during flow-induced atherogenesis in mouse carotid artery: An expanded flow cytometry method. Arterioscler Thromb Vasc Biol. 2012;32:623–632. doi: 10.1161/ATVBAHA.111.242180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jo H, Song H, Mowbray A. Role of nadph oxidases in disturbed flow- and bmp4-induced inflammation and atherosclerosis. Antioxid Redox Signal. 2006;8:1609–1619. doi: 10.1089/ars.2006.8.1609. [DOI] [PubMed] [Google Scholar]
- 28.Ni CW, Qiu H, Jo H. Microrna-663 upregulated by oscillatory shear stress plays a role in inflammatory response of endothelial cells. Am J Physiol Heart Circ Physiol. 2011;300:H1762–1769. doi: 10.1152/ajpheart.00829.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nissen SE, Tuzcu EM, Schoenhagen P, Crowe T, Sasiela WJ, Tsai J, Orazem J, Magorien RD, O’Shaughnessy C, Ganz P, Reversal of Atherosclerosis with Aggressive Lipid Lowering I Statin therapy, ldl cholesterol, c-reactive protein, and coronary artery disease. N Engl J Med. 2005;352:29–38. doi: 10.1056/NEJMoa042000. [DOI] [PubMed] [Google Scholar]
- 30.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E, Pravastatin or Atorvastatin E. Infection Therapy-Thrombolysis in Myocardial Infarction I C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 31.Hu H, Sung A, Zhao G, Shi L, Qiu D, Nishimura T, Kao PN. Simvastatin enhances bone morphogenetic protein receptor type ii expression. Biochem Biophys Res Commun. 2006;339:59–64. doi: 10.1016/j.bbrc.2005.10.187. [DOI] [PubMed] [Google Scholar]
- 32.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 33.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr., Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the committee on vascular lesions of the council on arteriosclerosis, american heart association. Circulation. 1995;92:1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 34.Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, Morrell NW. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type ii bone morphogenetic protein receptor. Circulation. 2002;105:1672–1678. doi: 10.1161/01.cir.0000012754.72951.3d. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi H, Goto N, Kojima Y, Tsuda Y, Morio Y, Muramatsu M, Fukuchi Y. Downregulation of type ii bone morphogenetic protein receptor in hypoxic pulmonary hypertension. American journal of physiology. Lung cellular and molecular physiology. 2006;290:L450–458. doi: 10.1152/ajplung.00206.2005. [DOI] [PubMed] [Google Scholar]
- 36.Cogan JD, Pauciulo MW, Batchman AP, Prince MA, Robbins IM, Hedges LK, Stanton KC, Wheeler LA, Phillips JA, 3rd, Loyd JE, Nichols WC. High frequency of bmpr2 exonic deletions/duplications in familial pulmonary arterial hypertension. American journal of respiratory and critical care medicine. 2006;174:590–598. doi: 10.1164/rccm.200602-165OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Koerner SK, Levy PC, Reid LM, Vriem CE, Williams GW. Primary pulmonary hypertension. A national prospective study. Annals of internal medicine. 1987;107:216–223. doi: 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- 38.Loyd JE, Butler MG, Foroud TM, Conneally PM, Phillips JA, 3rd, Newman JH. Genetic anticipation and abnormal gender ratio at birth in familial primary pulmonary hypertension. American journal of respiratory and critical care medicine. 1995;152:93–97. doi: 10.1164/ajrccm.152.1.7599869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaine SP, Rubin LJ. Primary pulmonary hypertension. Lancet. 1998;352:719–725. doi: 10.1016/S0140-6736(98)02111-4. [DOI] [PubMed] [Google Scholar]
- 40.Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, Gibbs S, Lebrec D, Speich R, Beghetti M, Rich S, Fishman A. Clinical classification of pulmonary hypertension. Journal of the American College of Cardiology. 2004;43:5S–12S. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 41.McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006;114:1417–1431. doi: 10.1161/CIRCULATIONAHA.104.503540. [DOI] [PubMed] [Google Scholar]
- 42.McGoon M, Gutterman D, Steen V, Barst R, McCrory DC, Fortin TA, Loyd JE. Screening, early detection, and diagnosis of pulmonary arterial hypertension: Accp evidence-based clinical practice guidelines. Chest. 2004;126:14S–34S. doi: 10.1378/chest.126.1_suppl.14S. [DOI] [PubMed] [Google Scholar]
- 43.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351:1425–1436. doi: 10.1056/NEJMra040291. [DOI] [PubMed] [Google Scholar]
- 44.Chiu J-J, Chien S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong WK, Knowles JA, Morse JH. Bone morphogenetic protein receptor type ii c-terminus interacts with c-src: Implication for a role in pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2005;33:438–446. doi: 10.1165/rcmb.2005-0103OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hassel S, Eichner A, Yakymovych M, Hellman U, Knaus P, Souchelnytskyi S. Proteins associated with type ii bone morphogenetic protein receptor (bmpr-ii) and identified by two-dimensional gel electrophoresis and mass spectrometry. Proteomics. 2004;4:1346–1358. doi: 10.1002/pmic.200300770. [DOI] [PubMed] [Google Scholar]
- 47.Lavery K, Swain P, Falb D, Alaoui-Ismaili MH. Bmp-2/4 and bmp-6/7 differentially utilize cell surface receptors to induce osteoblastic differentiation of human bone marrow-derived mesenchymal stem cells. J Biol Chem. 2008;283:20948–20958. doi: 10.1074/jbc.M800850200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.David L, Mallet C, Keramidas M, Lamande N, Gasc JM, Dupuis-Girod S, Plauchu H, Feige JJ, Bailly S. Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ Res. 2008;102:914–922. doi: 10.1161/CIRCRESAHA.107.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nasim MT, Ogo T, Chowdhury HM, Zhao L, Chen CN, Rhodes C, Trembath RC. Bmpr-ii deficiency elicits pro-proliferative and anti-apoptotic responses through the activation of tgfbeta-tak1-mapk pathways in pah. Human molecular genetics. 2012;21:2548–2558. doi: 10.1093/hmg/dds073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.