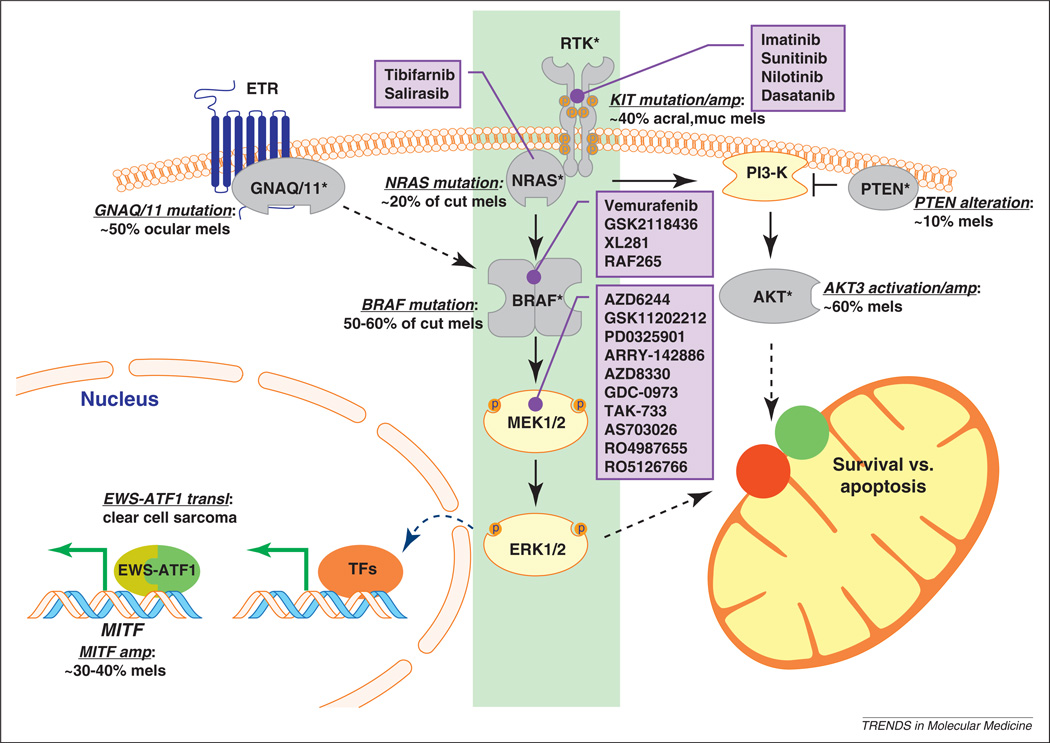
Figure 1.
Key mutational and therapeutic targets in melanoma. The RAS signaling network is rife with cancer-associated mutations. BRAF is the most commonly activated oncogene in cutaneous melanomas (cut mels), followed by NRAS. Upstream of RAS, the c-KIT receptor tyrosine kinase (RTK) is amplified or activated in a substantial fraction of acral and mucosal melanomas. It thus seems that the RTK–NRAS–BRAF–MEK–ERK cascade represents a central axis (highlighted in green) that is activated in nearly all melanomas. Parallel to this axis is the PI3-K pathway, which is also activated in melanomas either through loss of PTEN or activation of AKT3. In addition, it has recently been shown that GNAQ and GNA11, which are transducers of the endothelin receptor signal (ETR), are mutated in ocular melanomas. These heightened signaling events lead to both increased transcription of survival genes (such as MITF) and enhanced pro-survival factors in mitochondria through the regulation of BCL2 family proteins (red, pro-apoptotic; green, pro-survival). MITF, which is a master regulator of melanocyte survival, is also amplified in melanomas and a target of the EWS-ATF1 fusion protein described in clear cell sarcomas (so-called melanoma of the soft parts). Drugs known to inhibit the central axis and with a potential therapeutic impact on melanoma are listed in the purple boxes.