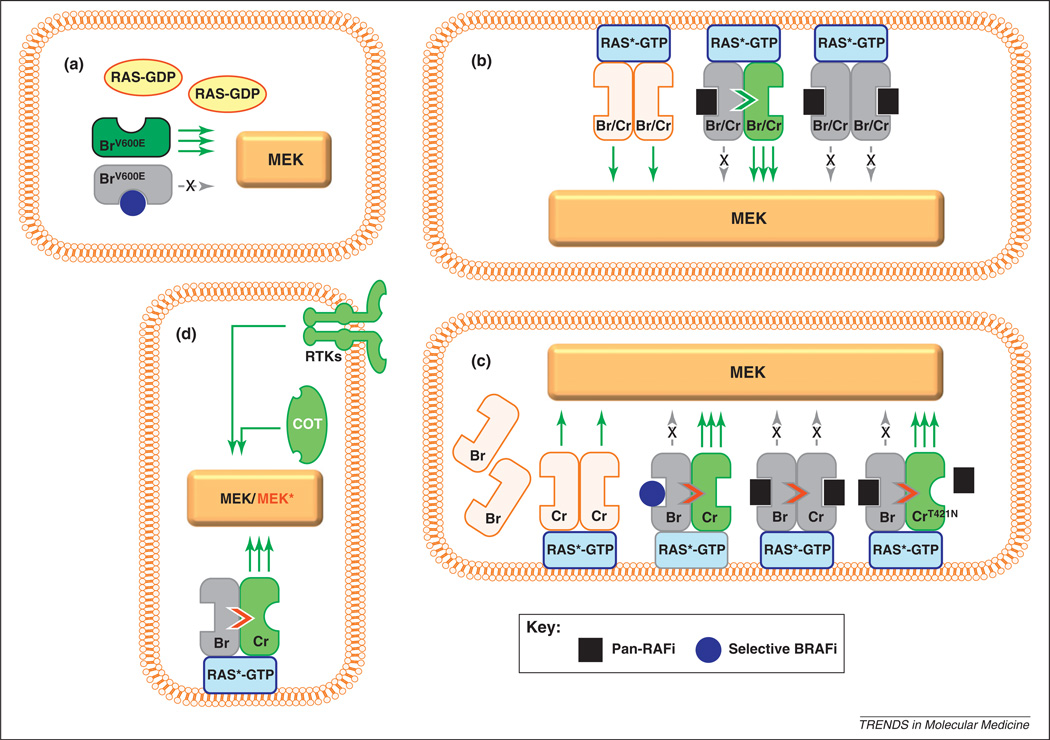
Figure 3.
Models of RAF activation and inhibition, (a) In cells with mutated BRAF (BrV600E), RAS is inactive, cytoplasmic and unable to induce RAF dimerization. MEK phosphorylation and activation occur almost exclusively via BRAFV600E and thus are effectively silenced by a selective BRAF inhibitor (blue circle). This would explain the dramatic tumor shrinkage early in treatment with vemurafenib. In some cells with wild-type BRAF, RAS is activated (RAS*-GTP), presumably by mutagenesis (e.g. NRAS mutations) or other upstream receptor events. There are at least two models of signal transduction in these RAS-stimulated tumors. In the transactivation model (b), active RAS*-GTP mobilizes to the membrane and induces homodimerization or heterodimerization of wild-type BRAF and CRAF proteins (Br/Cr), which in turn initiates signaling. At low concentrations, an ATP-competitive RAF inhibitor may occupy the active site of one of the RAF protomers. Inhibitor binding to the active site of one RAF kinase induces a conformational change that facilitates transactivation of the other RAF molecule in the dimer; therefore, there is paradoxical enhancement of MEK/ERK signaling through the uninhibited partner. At higher inhibitor concentrations, both RAF molecules are inhibited and MEK/ERK signaling is completely abolished. In the translocation model (c), wild-type BRAF (Br) remains largely inactive in the cytoplasm owing to autoinhibitory signals. Activated RAS*-GTP triggers MAPK signaling through CRAF (Cr). When wild-type BRAF binds a selective ATP-competitive BRAF inhibitor, the BRAF molecule is recruited to the membrane, thereby enhancing the RAS*-GTP/CRAF interaction and signaling (as in b). With pan-RAF inhibitors, wild-type BRAF may still enhance the RAS*-GTP/CRAF interaction, but MEK/ERK signaling is suppressed because CRAF activity itself is concurrently abrogated by inhibitor binding. In situations in which CRAF harbors a gatekeeper mutation, such as p.Thr421Asn (CrT421N), a pan-RAF agent inhibits BRAF, which then transactivates CRAFT421N; however, CRAFT421N is not inhibited by the pan-RAF agent because the mutation prevents drug binding. Both models suggest mechanisms by which BRAF inhibition may lead to increased stimulation of CRAF in RAS-mutated cells or growth-factor-stimulated cells, (d) Mechanisms of BRAF inhibitor resistance include activation of receptor tyrosine kinases (RTKs) such as PDGFR and IGF1R, CRAF and other protein kinases (e.g. COT), along with mutational activation of NRAS and MEK (MEK*).