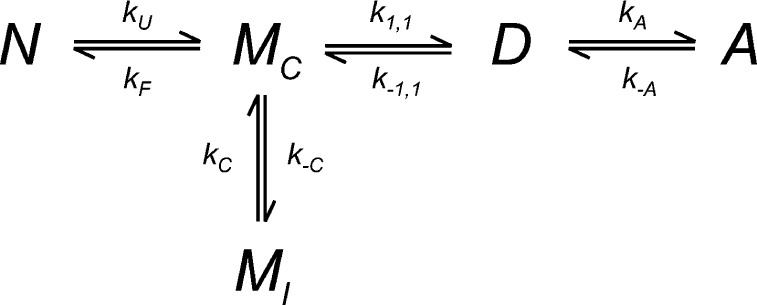
Figure 5.
The proposed reaction mechanism for amorphous aggregation of I27 in 28% TFE. N is the natively folded protein, MC is the aggregation-competent unfolded monomer, and MI is an unfolded state, which is not aggregation-competent (incompetent). D is a dimer, formed from two units of MC, and A is aggregate. kU and kF are the rate constants for unfolding and folding of N; k1,1 and k–1,1 are the forward and reverse rate constants for dimerization of MC to D; kA and k–A are the forward and reverse rate constants for aggregation (of D); and kC and k–C are the forward and reverse rate constants for the conversion of MI to MC.