Table 2. Analogues Containing Disubstituted Phenyl Rings.
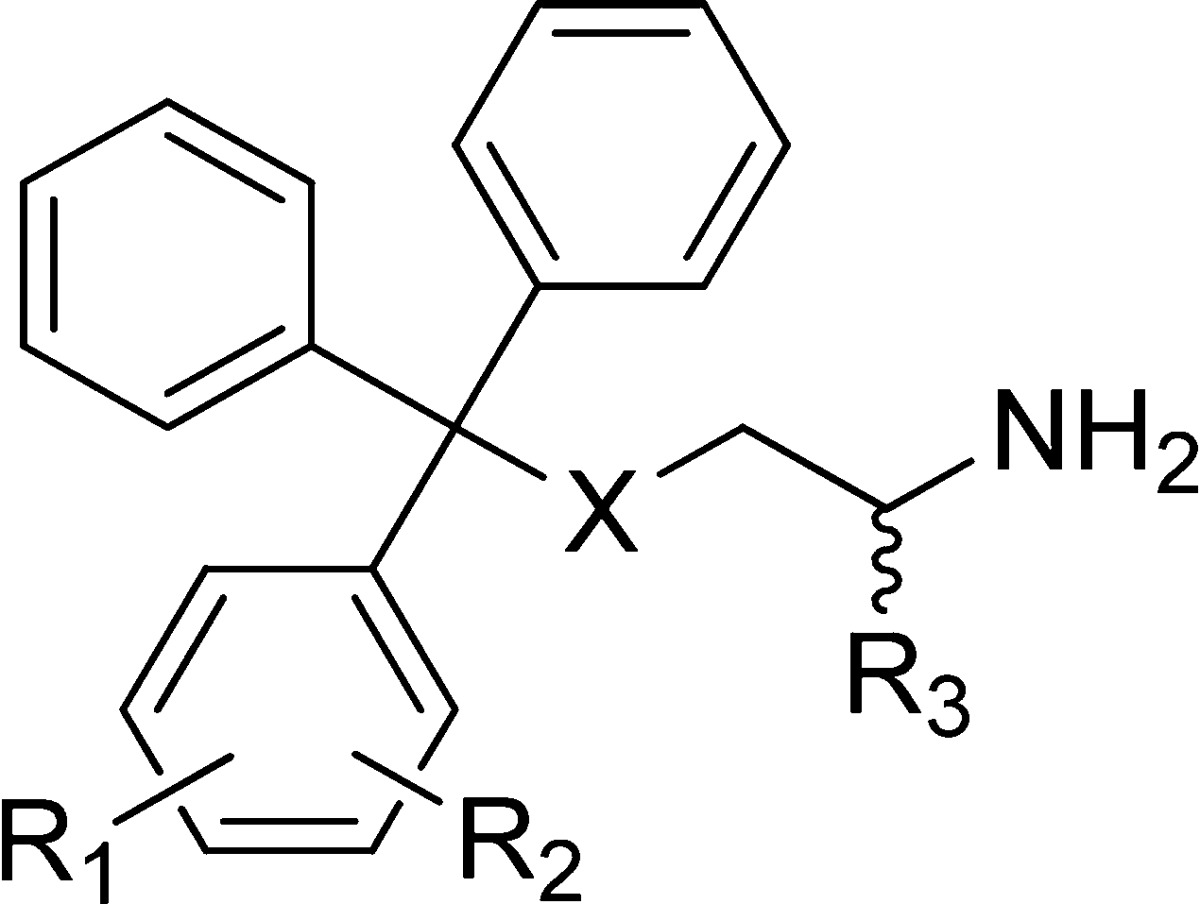
| compd | X | R1 | R2 | R3 | inhibition of basal ATPase activity Kiapp (nM) | LE | K562 cells GI50 (nM) |
|---|---|---|---|---|---|---|---|
| 28 | S | 2-F | 3-Me | H | 293.6 ± 23.2 | 0.36 | 2547 ± 141 |
| 29 | S | 2-F | 4-Me | H | 201.3 ± 18.7 | 0.37 | 2084 ± 109 |
| 30 | S | 2-F | 4-OMe | (R)-CO2H | 10.4 ± 4.5 | 0.38 | 82 ± 3 |
| 31 | S | 2-F | 4-OMe | H | 11.6 ± 3.7 | 0.42 | 489 ± 26 |
| 32 | S | 3-F | 4-OMe | H | 162.2 ± 15.6 | 0.36 | 1892 ± 134 |
| 33 | S | 3-Cl | 4-Cl | H | 35.2 ± 4.9 | 0.41 | 1993 ± 343 |
| 34 | S | 3-Me | 4-Me | (R)-CO2H | 1.2 ± 0.1 | 0.43 | 72 ± 8 |
| 35 | S | 3-Me | 4-Me | H | 25.7 ± 6.3 | 0.41 | 729 ± 43 |
| 36 | S | 3-Et | 4-Me | (R)-CO2H | 4.6 ± 1.7 | 0.39 | 34 ± 2 |
| 37 | S | 3-Et | 4-Me | H | 7.8 ± 3.9 | 0.43 | 1045 ± 42 |
| 38 | S | 3,4-(CH2)4 | (R)-CO2H | 2.1 ± 0.5 | 0.40 | 56 ± 2 | |
| 39 | S | 3,4-(CH2)4 | H | 10.6 ± 3.2 | 0.40 | 934 ± 127 | |
| 40 | C | 2-F | 4-OMe | H | 37.9 ± 4.7 | 0.39 | 764 ± 42 |
| 41 | C | 3-Cl | 4-Cl | H | 31.4 ± 6.4 | 0.41 | 633 ± 126 |
| rac-42 | C | 3-Me | 4-Me | CO2H | 12.4 ± 4.4 | 0.38 | 23.4 ± 1.8 |
